Precision Medicine to Redefine Insulin Secretion and Monogenic Diabetes-Randomized Controlled Trial (PRISM-RCT) in Chinese patients with young-onset diabetes: design, methods and baseline characteristics
- PMID: 38901858
- PMCID: PMC11212116
- DOI: 10.1136/bmjdrc-2024-004120
Precision Medicine to Redefine Insulin Secretion and Monogenic Diabetes-Randomized Controlled Trial (PRISM-RCT) in Chinese patients with young-onset diabetes: design, methods and baseline characteristics
Abstract
Introduction: We designed and implemented a patient-centered, data-driven, holistic care model with evaluation of its impacts on clinical outcomes in patients with young-onset type 2 diabetes (T2D) for which there is a lack of evidence-based practice guidelines.
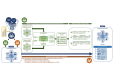
Research design and methods: In this 3-year Precision Medicine to Redefine Insulin Secretion and Monogenic Diabetes-Randomized Controlled Trial, we evaluate the effects of a multicomponent care model integrating use of information and communication technology (Joint Asia Diabetes Evaluation (JADE) platform), biogenetic markers and patient-reported outcome measures in patients with T2D diagnosed at ≤40 years of age and aged ≤50 years. The JADE-PRISM group received 1 year of specialist-led team-based management using treatment algorithms guided by biogenetic markers (genome-wide single-nucleotide polymorphism arrays, exome-sequencing of 34 monogenic diabetes genes, C-peptide, autoantibodies) to achieve multiple treatment goals (glycated hemoglobin (HbA1c) <6.2%, blood pressure <120/75 mm Hg, low-density lipoprotein-cholesterol <1.2 mmol/L, waist circumference <80 cm (women) or <85 cm (men)) in a diabetes center setting versus usual care (JADE-only). The primary outcome is incidence of all diabetes-related complications.
Results: In 2020-2021, 884 patients (56.6% men, median (IQR) diabetes duration: 7 (3-12) years, current/ex-smokers: 32.5%, body mass index: 28.40±5.77 kg/m2, HbA1c: 7.52%±1.66%, insulin-treated: 27.7%) were assigned to JADE-only (n=443) or JADE-PRISM group (n=441). The profiles of the whole group included positive family history (74.7%), general obesity (51.4%), central obesity (79.2%), hypertension (66.7%), dyslipidemia (76.4%), albuminuria (35.4%), estimated glomerular filtration rate <60 mL/min/1.73 m2 (4.0%), retinopathy (13.8%), atherosclerotic cardiovascular disease (5.2%), cancer (3.1%), emotional distress (26%-38%) and suboptimal adherence (54%) with 5-item EuroQol for Quality of Life index of 0.88 (0.87-0.96). Overall, 13.7% attained ≥3 metabolic targets defined in secondary outcomes. In the JADE-PRISM group, 4.5% had pathogenic/likely pathogenic variants of monogenic diabetes genes; 5% had autoantibodies and 8.4% had fasting C-peptide <0.2 nmol/L. Other significant events included low/large birth weight (33.4%), childhood obesity (50.7%), mental illness (10.3%) and previous suicide attempts (3.6%). Among the women, 17.3% had polycystic ovary syndrome, 44.8% required insulin treatment during pregnancy and 17.3% experienced adverse pregnancy outcomes.
Conclusions: Young-onset diabetes is characterized by complex etiologies with comorbidities including mental illness and lifecourse events.
Trial registration number: NCT04049149.
Keywords: MODY; genetics; latent autoimmune diabetes in adults; randomized controlled trials as topic.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JCNC and RCWM hold patents for using genetic markers to predict diabetes and its complications for personalized care. JCNC, RCWM and CL are cofounders of a start-up biotech company partially supported by the Technology Start-up Support Scheme for Universities (TSSSU) of the Hong Kong Government Innovation and Technology Commission.
Figures

References
-
- Chan JCN, Elaine C, Kong A, et al. . Multifaceted nature of young-onset diabetes - can Genomic medicine improve the precision of diagnosis and management J Transl Genet Genom 2024;8:13–34. 10.20517/jtgg.2023.36 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
