Impact of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in children with cystic fibrosis and one or two F508del alleles
- PMID: 38901883
- PMCID: PMC11375515
- DOI: 10.1183/13993003.00004-2024
Impact of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in children with cystic fibrosis and one or two F508del alleles
Abstract
Background: We recently demonstrated that elexacaftor/tezacaftor/ivacaftor (ETI) improves the lung clearance index (LCI) and abnormalities in lung morphology detected by magnetic resonance imaging (MRI) in adolescent and adult patients with cystic fibrosis (CF). However, real-world data on the effect of ETI on these sensitive outcomes of lung structure and function in school-age children with CF have not been reported. The aim of this study was therefore to examine the effect of ETI on the LCI and the lung MRI score in children aged 6-11 years with CF and one or two F508del alleles.
Methods: This prospective, observational, multicentre, post-approval study assessed the longitudinal LCI up to 12 months and the lung MRI score before and 3 months after initiation of ETI.
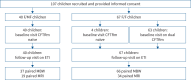
Results: A total of 107 children with CF including 40 heterozygous for F508del and a minimal function mutation (F/MF) and 67 homozygous for F508del (F/F) were enrolled in this study. Treatment with ETI improved the median (interquartile range (IQR)) LCI in F/MF (-1.0 (-2.0- -0.1); p<0.01) and F/F children (-0.8 (-1.9- -0.2); p<0.001) from 3 months onwards. Further, ETI improved the median (IQR) MRI global score in F/MF (-4.0 (-9.0-0.0); p<0.01) and F/F children (-3.5 (-7.3- -0.8); p<0.001).
Conclusions: ETI improves early abnormalities in lung ventilation and morphology in school-age children with CF and at least one F508del allele in a real-world setting. Our results support early initiation of ETI to reduce or even prevent lung disease progression in school-age children with CF.
Copyright ©The authors 2024.
Conflict of interest statement
Conflicts of interest: M. Stahl, S.Y. Graeber and S. Thee are participants of the Berlin Institute of Health (BIH)-Charité Clinician Scientist Program, and J. Röhmel is participant of the Case Analysis and Decision Support (CADS) program funded by Charité – Universitätsmedizin Berlin and the BIH. M. Stahl reports an Independent Research Innovation Award and honoraria for lectures and participation in advisory boards, all by Vertex Pharmaceuticals Incorporated, outside of the submitted work; she is Chairman of the German CF Research Council (FGM), Treasurer of the German Society of Paediatric Pulmonology (GPP) and was Secretary of the Group CF of the Paediatric Assembly of the ERS. M. Dohna is a participant of the Ellen-Schmidt Habilitationsförderung funded by the Hannover Medical School. S.Y. Graeber reports grants from the German CF Foundation and Vertex Pharmaceuticals Incorporated, and honoraria from Chiesi GmbH and Vertex Pharmaceuticals Incorporated for lectures and participation in advisory boards, outside of the submitted work. O. Sommerburg reports grants and honoraria from Vertex Pharmaceuticals Incorporated for lectures, outside of the submitted work. S.T. Pallenberg is a member of the Else-Kröner Forschungskolleg TITUS. A. Voskrebenzev reports a grant and honoraria for lectures from Siemens Healthineers, outside of the submitted work, holds a patent for a method of quantitative magnetic resonance lung imaging (Voskrebenzev, Gutberlet, Vogel-Claussen; number EP3107066, US-2016-0367200-Al 22.12.2016), and is a stockholder and CEO of BioVisioneers GmbH. K. Schütz reports payments for attending meetings and/or travel from Vertex Pharmaceuticals Incorporated, outside of the submitted work. G. Hansen reports receipt of consultation fees from Sanofi GmbH, outside of the submitted work. F. Doellinger reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Bayer Vital, Berlin-Chemie Menarini, Boehringer Ingelheim and Chiesi GmbH, payment for expert testimony from Calyx, and support for attending meetings from Bayer. E. Steinke reports grants from Berlin Institute of Health at Charité Berlin, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Pharmaceuticals Incorporated. S. Thee reports honoraria for lectures and payment for attending meetings and/or travel from Vertex Pharmaceuticals Incorporated and Viatris, outside of the submitted work. J. Röhmel reports honoraria for lectures from Vertex Pharmaceuticals Incorporated, outside the submitted work; additionally, he is work package leader in BEAT-PCD (ERS-CRC). M.O. Wielpütz reports a grant from Vertex Pharmaceuticals Incorporated, and receipt of consulting fees and honoraria for lectures from Vertex Pharmaceuticals Incorporated and Boehringer Ingelheim, outside of the submitted work. L. Naehrlich reports receipt of fees for a data quality project of the German CF Registry. He is the medical lead of the German CF Registry, the pharmacovigilance study manager of the European Cystic Fibrosis Society Patient Registry and part of the Trial Steering Committee for CF STORM. He also reports grants from the German Center for Lung Research, Vertex Pharmaceuticals and Mukoviszidose Institute, and receipt of medical writing services from Articulate Science. J. Vogel-Claussen reports grants from BMBF, Siemens Healthineers, AstraZeneca, Boehringer Ingelheim and GSK, royalties or licenses from Siemens Healthineers, receipt of consulting fees from AstraZeneca, honoraria for lectures from Siemens Healthineers, AstraZeneca, Boehringer Ingelheim, GSK, Roche, Coreline Soft and Bayer, payments for attending meetings and/or travel from Vertex Pharmaceuticals Incorporated, Bayer, GSK and AstraZeneca, and holds a patent for a method of quantitative magnetic resonance lung imaging (Voskrebenzev, Gutberlet, Vogel-Claussen; number EP3107066, US-2016-0367200-Al 22.12.2016). B. Tümmler reports support for the present study from Bundesministerium für Forschung und Technologie, grants from the German Research Foundation (DFG; CRC 900; Excellence cluster “RESIST”), consultancy fees from Helmholtz Institut für Infektionsforschung, payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Pharmaceutical (Germany) Incorporated, participation on a data and safety monitoring board or advisory board with Vertex Pharmaceuticals Incorporated, and leadership roles with Christiane Herzog Stiftung and the Microbiome/Metagenome Group of the German Center for Lung Research (DZL). M.A. Mall reports grants from the German Research Foundation (DFG; SFB-TR 84, and project 450557679) and the German Innovation Fund (01NVF19008), outside of the submitted work. Additionally, he reports receipt of consulting fees from AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Santhera, Splisense and Vertex Pharmaceuticals Incorporated, of honoraria for lectures from Vertex Pharmaceuticals Incorporated and participation in advisory boards from AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Pari and Vertex Pharmaceuticals Incorporated, and of payment for travel from Vertex Pharmaceuticals Incorporated and Boehringer Ingelheim, all outside of the submitted work. He is a Fellow of ERS (FERS). A-M. Dittrich reports support for the present study from the German Center for Lung Research (DZL), Vertex Pharmaceuticals Incorporated and European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN), grants from Vertex Pharmaceuticals Incorporated, ECFS-CTN, DFG and Christiane Herzog Stiftung, consultancy fees from the c4c consortium, GSK and European Cystic Fibrosis Society. The remaining authors have no potential conflicts of interest to disclose.
Figures






Comment in
-
The younger, the better: lessons learned from real-world studies on CFTR modulators in young children.Eur Respir J. 2024 Sep 5;64(3):2401178. doi: 10.1183/13993003.01178-2024. Print 2024 Sep. Eur Respir J. 2024. PMID: 39237313 No abstract available.
References
-
- Griese M, Costa S, Linnemann RW, et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for 24 weeks or longer in people with cystic fibrosis and one or more F508del alleles: interim results of an open-label phase 3 clinical trial. Am J Respir Crit Care Med 2021; 203: 381–385. doi: 10.1164/rccm.202008-3176LE - DOI - PMC - PubMed
-
- Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940–1948. doi: 10.1016/S0140-6736(19)32597-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
