Multiple roles for hypoxia inducible factor 1-alpha in airway epithelial cells during mucormycosis
- PMID: 38902255
- PMCID: PMC11190229
- DOI: 10.1038/s41467-024-49637-8
Multiple roles for hypoxia inducible factor 1-alpha in airway epithelial cells during mucormycosis
Abstract
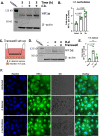
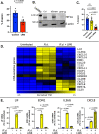
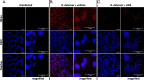
During pulmonary mucormycosis, inhaled sporangiospores adhere to, germinate, and invade airway epithelial cells to establish infection. We provide evidence that HIF1α plays dual roles in airway epithelial cells during Mucorales infection. We observed an increase in HIF1α protein accumulation and increased expression of many known HIF1α-responsive genes during in vitro infection, indicating that HIF1α signaling is activated by Mucorales infection. Inhibition of HIF1α signaling led to a substantial decrease in the ability of R. delemar to invade cultured airway epithelial cells. Transcriptome analysis revealed that R. delemar infection induces the expression of many pro-inflammatory genes whose expression was significantly reduced by HIF1α inhibition. Importantly, pharmacological inhibition of HIF1α increased survival in a mouse model of pulmonary mucormycosis without reducing fungal burden. These results suggest that HIF1α plays two opposing roles during mucormycosis: one that facilitates the ability of Mucorales to invade the host cells and one that facilitates the ability of the host to mount an innate immune response.
© 2024. The Author(s).
Conflict of interest statement
A.S.I. owns shares in Vitalex Biosciences, a start-up company that is developing immunotherapies and diagnostics for mucormycosis. T.J.W. has received grants for experimental and clinical antimicrobial pharmacology, therapeutics, and diagnostics to his institution from Amplyx, Astellas, Gilead, Lediant, Merck, Scynexis, Shionogi, T2 Biosystems, Viosera; and has served as a consultant to Abbott, Astellas, Karyopharm, Leadiant, Partner Therapeutics, Scynexis, Shionogi, Statera, and T2 Biosystems. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- AI141360/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- AI063503/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- R01 AI063503/AI/NIAID NIH HHS/United States
- U19 AI110820/AI/NIAID NIH HHS/United States
- R01 AI141360/AI/NIAID NIH HHS/United States

