Multiomic analysis identifies a high-risk signature that predicts early clinical failure in DLBCL
- PMID: 38902256
- PMCID: PMC11189905
- DOI: 10.1038/s41408-024-01080-0
Multiomic analysis identifies a high-risk signature that predicts early clinical failure in DLBCL
Abstract
Recent genetic and molecular classification of DLBCL has advanced our knowledge of disease biology, yet were not designed to predict early events and guide anticipatory selection of novel therapies. To address this unmet need, we used an integrative multiomic approach to identify a signature at diagnosis that will identify DLBCL at high risk of early clinical failure. Tumor biopsies from 444 newly diagnosed DLBCL were analyzed by WES and RNAseq. A combination of weighted gene correlation network analysis and differential gene expression analysis was used to identify a signature associated with high risk of early clinical failure independent of IPI and COO. Further analysis revealed the signature was associated with metabolic reprogramming and identified cases with a depleted immune microenvironment. Finally, WES data was integrated into the signature and we found that inclusion of ARID1A mutations resulted in identification of 45% of cases with an early clinical failure which was validated in external DLBCL cohorts. This novel and integrative approach is the first to identify a signature at diagnosis, in a real-world cohort of DLBCL, that identifies patients at high risk for early clinical failure and may have significant implications for design of therapeutic options.
© 2024. The Author(s).
Conflict of interest statement
AJN: Research funding from BMS. MES, MO, NS, CCH, and AKG: employed by BMS.
Figures


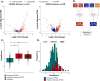
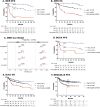
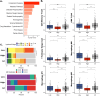

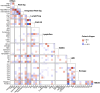
Update of
-
Multiomic Analysis Identifies a High-Risk Metabolic and TME Depleted Signature that Predicts Early Clinical Failure in DLBCL.medRxiv [Preprint]. 2023 Jun 10:2023.06.07.23290748. doi: 10.1101/2023.06.07.23290748. medRxiv. 2023. Update in: Blood Cancer J. 2024 Jun 20;14(1):100. doi: 10.1038/s41408-024-01080-0. PMID: 37333387 Free PMC article. Updated. Preprint.
References
-
- Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–73. doi: 10.1200/JCO.2013.51.5866. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32 AI170478/AI/NIAID NIH HHS/United States
- U01 CA195568/CA/NCI NIH HHS/United States
- SPORE-P50 CA97274/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- P30 CA086862/CA/NCI NIH HHS/United States
- R01 CA212162-01A1/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

