Distinct dynamics of mRNA LNPs in mice and nonhuman primates revealed by in vivo imaging
- PMID: 38902327
- PMCID: PMC11189915
- DOI: 10.1038/s41541-024-00900-5
Distinct dynamics of mRNA LNPs in mice and nonhuman primates revealed by in vivo imaging
Abstract
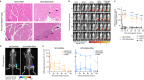
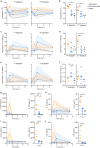
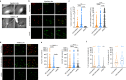
The characterization of vaccine distribution to relevant tissues after in vivo administration is critical to understanding their mechanisms of action. Vaccines based on mRNA lipid nanoparticles (LNPs) are now being widely considered against infectious diseases and cancer. Here, we used in vivo imaging approaches to compare the trafficking of two LNP formulations encapsulating mRNA following intramuscular administration: DLin-MC3-DMA (MC3) and the recently developed DOG-IM4. The mRNA formulated in DOG-IM4 LNPs persisted at the injection site, whereas mRNA formulated in MC3 LNPs rapidly migrated to the draining lymph nodes. Furthermore, MC3 LNPs induced the fastest increase in blood neutrophil counts after injection and greater inflammation, as shown by IL-1RA, IL-15, CCL-1, and IL-6 concentrations in nonhuman primate sera. These observations highlight the influence of the nature of the LNP on mRNA vaccine distribution and early immune responses.
© 2024. The Author(s).
Conflict of interest statement
K.L., V.P., M.R., J.H. and C.P. are Sanofi employees or were under contract with Sanofi at the time of the study and may hold shares and/or stock options in the company. The remaining authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

