Coordinated action of a gut-liver pathway drives alcohol detoxification and consumption
- PMID: 38902331
- PMCID: PMC12475873
- DOI: 10.1038/s42255-024-01063-2
Coordinated action of a gut-liver pathway drives alcohol detoxification and consumption
Abstract
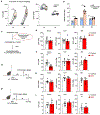
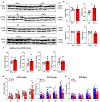
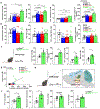
Alcohol use disorder (AUD) affects millions of people worldwide, causing extensive morbidity and mortality with limited pharmacological treatments. The liver is considered as the principal site for the detoxification of ethanol metabolite, acetaldehyde (AcH), by aldehyde dehydrogenase 2 (ALDH2) and as a target for AUD treatment, however, our recent data indicate that the liver only plays a partial role in clearing systemic AcH. Here we show that a liver-gut axis, rather than liver alone, synergistically drives systemic AcH clearance and voluntary alcohol drinking. Mechanistically, we find that after ethanol intake, a substantial proportion of AcH generated in the liver is excreted via the bile into the gastrointestinal tract where AcH is further metabolized by gut ALDH2. Modulating bile flow significantly affects serum AcH level and drinking behaviour. Thus, combined targeting of liver and gut ALDH2, and manipulation of bile flow and secretion are potential therapeutic strategies to treat AUD.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures








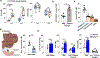






References
-
- Goedde HW, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Human Genetics 88, 344–346 (1992). - PubMed
-
- Lai C-L, et al. Dominance of the Inactive Asian Variant Over Activity and Protein Contents of Mitochondrial Aldehyde Dehydrogenase 2 in Human Liver. Alcohol: Clinical and Experimental Research 38, 44–50 (2014). - PubMed
-
- Higuchi S, et al. Aldehyde dehydrogenase genotypes In Japanese alcoholics. The Lancet 343, 741–742 (1994). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

