Exportin 1 governs the immunosuppressive functions of myeloid-derived suppressor cells in tumors through ERK1/2 nuclear export
- PMID: 38902348
- PMCID: PMC11291768
- DOI: 10.1038/s41423-024-01187-1
Exportin 1 governs the immunosuppressive functions of myeloid-derived suppressor cells in tumors through ERK1/2 nuclear export
Abstract
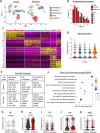
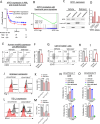
Myeloid-derived suppressor cells (MDSCs) are a main driver of immunosuppression in tumors. Understanding the mechanisms that determine the development and immunosuppressive function of these cells could provide new therapeutic targets to improve antitumor immunity. Here, using preclinical murine models, we discovered that exportin 1 (XPO1) expression is upregulated in tumor MDSCs and that this upregulation is induced by IL-6-induced STAT3 activation during MDSC differentiation. XPO1 blockade transforms MDSCs into T-cell-activating neutrophil-like cells, enhancing the antitumor immune response and restraining tumor growth. Mechanistically, XPO1 inhibition leads to the nuclear entrapment of ERK1/2, resulting in the prevention of ERK1/2 phosphorylation following the IL-6-mediated activation of the MAPK signaling pathway. Similarly, XPO1 blockade in human MDSCs induces the formation of neutrophil-like cells with immunostimulatory functions. Therefore, our findings revealed a critical role for XPO1 in MDSC differentiation and suppressive functions; exploiting these new discoveries revealed new targets for reprogramming immunosuppressive MDSCs to improve cancer therapeutic responses.
Keywords: Exportin 1; MAPK pathway; MDSCs; Tumor.
© 2024. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
Advisory Board/Consulting for P.L.M.: BlueBird Biotech, Bristol-Myers Squibb, Celgene, Fate Therapeutics, Janssen, Juno, Karyopharm, Magenta Therapeutics, Sanofi, and Takeda. Honoraria for P.L.M.: BlueBird Biotech, Bristol-Myers Squibb, Celgene, Fate Therapeutics, Janssen, Juno, Karyopharm, Magenta Therapeutics, Sanofi, and Takeda. Employment for Y.L.: Karyopharm.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

