Abundant extrasynaptic expression of α3β4-containing nicotinic acetylcholine receptors in the medial habenula-interpeduncular nucleus pathway in mice
- PMID: 38902419
- PMCID: PMC11189931
- DOI: 10.1038/s41598-024-65076-3
Abundant extrasynaptic expression of α3β4-containing nicotinic acetylcholine receptors in the medial habenula-interpeduncular nucleus pathway in mice
Abstract
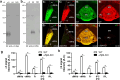
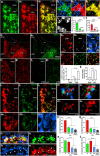
Nicotinic acetylcholine receptors (nAChRs) in the medial habenula (MHb)-interpeduncular nucleus (IPN) pathway play critical roles in nicotine-related behaviors. This pathway is particularly enriched in nAChR α3 and β4 subunits, both of which are genetically linked to nicotine dependence. However, the cellular and subcellular expression of endogenous α3β4-containing nAChRs remains largely unknown because specific antibodies and appropriate detection methods were unavailable. Here, we successfully uncovered the expression of endogenous nAChRs containing α3 and β4 subunits in the MHb-IPN pathway using novel specific antibodies and a fixative glyoxal that enables simultaneous detection of synaptic and extrasynaptic molecules. Immunofluorescence and immunoelectron microscopy revealed that both subunits were predominantly localized to the extrasynaptic cell surface of somatodendritic and axonal compartments of MHb neurons but not at their synaptic junctions. Immunolabeling for α3 and β4 subunits disappeared in α5β4-knockout brains, which we used as negative controls. The enriched and diffuse extrasynaptic expression along the MHb-IPN pathway suggests that α3β4-containing nAChRs may enhance the excitability of MHb neurons and neurotransmitter release from their presynaptic terminals in the IPN. The revealed distribution pattern provides a molecular and anatomical basis for understanding the functional role of α3β4-containing nAChRs in the crucial pathway of nicotine dependence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
α3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake.J Neurosci. 2021 Feb 24;41(8):1779-1787. doi: 10.1523/JNEUROSCI.0127-19.2020. Epub 2020 Dec 30. J Neurosci. 2021. PMID: 33380469 Free PMC article.
-
Role of β4* Nicotinic Acetylcholine Receptors in the Habenulo-Interpeduncular Pathway in Nicotine Reinforcement in Mice.Neuropsychopharmacology. 2016 Jun;41(7):1790-802. doi: 10.1038/npp.2015.346. Epub 2015 Nov 20. Neuropsychopharmacology. 2016. PMID: 26585290 Free PMC article.
-
The habenulo-interpeduncular pathway in nicotine aversion and withdrawal.Neuropharmacology. 2015 Sep;96(Pt B):213-22. doi: 10.1016/j.neuropharm.2014.11.019. Epub 2014 Dec 2. Neuropharmacology. 2015. PMID: 25476971 Free PMC article. Review.
-
Chronic Nicotine Exposure Alters the Neurophysiology of Habenulo-Interpeduncular Circuitry.J Neurosci. 2019 May 29;39(22):4268-4281. doi: 10.1523/JNEUROSCI.2816-18.2019. Epub 2019 Mar 13. J Neurosci. 2019. PMID: 30867261 Free PMC article.
-
Contribution of Variants in CHRNA5/A3/B4 Gene Cluster on Chromosome 15 to Tobacco Smoking: From Genetic Association to Mechanism.Mol Neurobiol. 2016 Jan;53(1):472-484. doi: 10.1007/s12035-014-8997-x. Epub 2014 Dec 5. Mol Neurobiol. 2016. PMID: 25471942 Review.
Cited by
-
Preferential Localization of STIM1 to dendritic subsurface ER structures in Mouse Purkinje Cells.J Neurosci. 2025 Mar 14;45(16):e1829242025. doi: 10.1523/JNEUROSCI.1829-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40086872
-
Calcium-activated ion channels drive atypical inhibition in medial habenula neurons.Sci Adv. 2025 Mar 21;11(12):eadq2629. doi: 10.1126/sciadv.adq2629. Epub 2025 Mar 19. Sci Adv. 2025. PMID: 40106550 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 17KK0160/Ministry of Education, Culture, Sports, Science and Technology
- 21K06746/Ministry of Education, Culture, Sports, Science and Technology
- 22K06784/Ministry of Education, Culture, Sports, Science and Technology
- 20H05628/Ministry of Education, Culture, Sports, Science and Technology
- 20H05628/Ministry of Education, Culture, Sports, Science and Technology
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

