The in vitro dynamics of pseudo-vascular network formation
- PMID: 38902534
- PMCID: PMC11300916
- DOI: 10.1038/s41416-024-02722-7
The in vitro dynamics of pseudo-vascular network formation
Abstract
Background/objectives: Pseudo-vascular network formation in vitro is considered a key characteristic of vasculogenic mimicry. While many cancer cell lines form pseudo-vascular networks, little is known about the spatiotemporal dynamics of these formations.
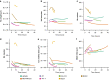
Methods: Here, we present a framework for monitoring and characterising the dynamic formation and dissolution of pseudo-vascular networks in vitro. The framework combines time-resolved optical microscopy with open-source image analysis for network feature extraction and statistical modelling. The framework is demonstrated by comparing diverse cancer cell lines associated with vasculogenic mimicry, then in detecting response to drug compounds proposed to affect formation of vasculogenic mimics. Dynamic datasets collected were analysed morphometrically and a descriptive statistical analysis model was developed in order to measure stability and dissimilarity characteristics of the pseudo-vascular networks formed.
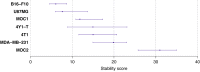
Results: Melanoma cells formed the most stable pseudo-vascular networks and were selected to evaluate the response of their pseudo-vascular networks to treatment with axitinib, brucine and tivantinib. Tivantinib has been found to inhibit the formation of the pseudo-vascular networks more effectively, even in dose an order of magnitude less than the two other agents.
Conclusions: Our framework is shown to enable quantitative analysis of both the capacity for network formation, linked vasculogenic mimicry, as well as dynamic responses to treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

