Target engagement and immunogenicity of an active immunotherapeutic targeting pathological α-synuclein: a phase 1 placebo-controlled trial
- PMID: 38902546
- PMCID: PMC11405261
- DOI: 10.1038/s41591-024-03101-8
Target engagement and immunogenicity of an active immunotherapeutic targeting pathological α-synuclein: a phase 1 placebo-controlled trial
Abstract
Investigational therapeutics that target toxic species of α-synuclein (αSyn) aim to slow down or halt disease progression in patients with Parkinson's disease (PD). Here this 44-week, randomized, placebo-controlled, double-blind, single-center phase 1 study investigated safety, tolerability and immunogenicity of UB-312, an active immunotherapeutic targeting pathological αSyn, in patients with PD. The primary outcome measures were adverse event frequency and change in anti-αSyn antibody titers in blood and cerebrospinal fluid (CSF). Exploratory outcomes were changes in clinical scales and biomarker-based target engagement as measured by seed amplification assays. Twenty patients were randomized 7:3 (UB-312:placebo) into 300/100/100 μg or 300/300/300 μg (weeks 1, 5 and 13) intramuscular prime-boost dose groups. Safety was similar across groups; adverse events were mostly mild and transient. Two patients experienced three serious adverse events in total, one possibly treatment related; all resolved without sequalae. Anti-αSyn antibodies in serum from 12/13 and CSF from 5/13 patients who received three UB-312 doses confirmed immunogenicity. Mean serum titers (in log-dilution factor) increased from baseline by 1.398 and 1.354, and peaked at week 29 at 2.520 and 2.133, for 300/100/100 μg and 300/300/300 μg, respectively. CSF titers were 0 at baseline and were 0.182 and 0.032 at week 21, respectively. Exploratory analyses showed no statistical differences in clinical scales but a significant reduction of αSyn seeds in CSF of a subset of UB-312-treated patients. These data support further UB-312 development. ClinicalTrials.gov: NCT04075318 .
© 2024. The Author(s).
Conflict of interest statement
P.E., P.K., I.R., M.V., M.L.d.K., M.F.J.M.V. and G.J.G. were employees of CHDR and conducted the study, and declare no competing interests. J.D.B., S.D., L.F., Y.-T.H., D.M., Y.S.S., M.M.V., H.J.Y. and J.-C.D. are employees at Vaxxinity, Inc, the study sponsor (or were at the time of the study) and hold company stock options. L.C.-M., C.M.F. and Y.M. are employees of Amprion Inc. L.C.-M., C.M.F., Y.M. and M.S. are named inventors of several patents and pending patent applications related to the αSyn-SAA. These patents and pending applications are either co-owned by Amprion with UT Health and exclusively licensed by Amprion Inc. or are owned solely by Amprion. The use of αSyn-SAA as described herein by Mayo Clinic is by limited permission only. All inquiries about the use of αSyn-SAA for diagnostic and/or drug development purposes should be directed to L.C.-M. P.M., G.S. and W.S. declare no competing interests.
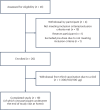
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

