Effects of Oral ALZ-801/Valiltramiprosate on Plasma Biomarkers, Brain Hippocampal Volume, and Cognition: Results of 2-Year Single-Arm, Open-Label, Phase 2 Trial in APOE4 Carriers with Early Alzheimer's Disease
- PMID: 38902571
- PMCID: PMC11289173
- DOI: 10.1007/s40265-024-02067-8
Effects of Oral ALZ-801/Valiltramiprosate on Plasma Biomarkers, Brain Hippocampal Volume, and Cognition: Results of 2-Year Single-Arm, Open-Label, Phase 2 Trial in APOE4 Carriers with Early Alzheimer's Disease
Abstract
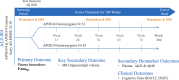
Introduction: ALZ-801/valiltramiprosate is a small-molecule oral inhibitor of beta amyloid (Aβ) aggregation and oligomer formation being studied in a phase 2 trial in APOE4 carriers with early Alzheimer's disease (AD) to evaluate treatment effects on fluid and imaging biomarkers and cognitive assessments.
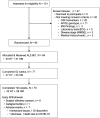
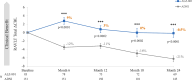
Methods: The single-arm, open-label phase 2 trial was designed to evaluate the effects of the ALZ-801 265 mg tablet taken twice daily (after 2 weeks once daily) on plasma fluid AD biomarkers, hippocampal volume (HV), and cognition over 104 weeks in APOE4 carriers. The study enrolled subjects aged 50-80 years, with early AD [Mini-Mental State Examination (MMSE) ≥ 22, Clinical Dementia Rating-Global (CDR-G) 0.5 or 1], apolipoprotein E4 (APOE4) genotypes including APOE4/4 and APOE3/4 genotypes, and positive cerebrospinal fluid (CSF) AD biomarkers or prior amyloid scans. The primary outcome was plasma p-tau181, HV evaluated by magnetic resonance imaging (MRI) was the key secondary outcome, and plasma Aβ42 and Aβ40 were the secondary biomarker outcomes. The cognitive outcomes were the Rey Auditory Verbal Learning Test and the Digit Symbol Substitution Test. Safety and tolerability evaluations included treatment-emergent adverse events and amyloid-related imaging abnormalities (ARIA). The study was designed and powered to detect 15% reduction from baseline in plasma p-tau181 at the 104-week endpoint. A sample size of 80 subjects provided adequate power to detect this difference at a significance level of 0.05 using a two-sided paired t-test.
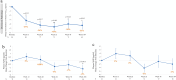
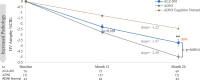
Results: The enrolled population of 84 subjects (31 homozygotes and 53 heterozygotes) was 52% females, mean age 69 years, MMSE 25.7 [70% mild cognitive impairment (MCI), 30% mild AD] with 55% on cholinesterase inhibitors. Plasma p-tau181 reduction from baseline was significant (31%, p = 0.045) at 104 weeks and all prior visits; HV atrophy was significantly reduced (p = 0.0014) compared with matched external controls from an observational Early AD study. Memory scores showed minimal decline from baseline over 104 weeks and correlated significantly with decreased HV atrophy (Spearman's 0.44, p = 0.002). Common adverse events were COVID infection and mild nausea, and no drug-related serious adverse events were reported. Of 14 early terminations, 6 were due to nonserious treatment-emergent adverse events and 1 death due to COVID. There was no vasogenic brain edema observed on MRI over 104 weeks.
Conclusions: The effect of ALZ-801 on reducing plasma p-tau181 over 2 years demonstrates target engagement and supports its anti-Aβ oligomer action that leads to a robust decrease in amyloid-induced brain neurodegeneration. The significant correlation between reduced HV atrophy and cognitive stability over 2 years suggests a disease-modifying effect of ALZ-801 treatment in patients with early AD. Together with the favorable safety profile with no events of vasogenic brain edema, these results support further evaluation of ALZ-801 in a broader population of APOE4 carriers, who represent two-thirds of patients with AD.
Trial registration: https://clinicaltrials.gov/study/NCT04693520 .
© 2024. The Author(s).
Conflict of interest statement
J.A.H., S.A.S., P.K., A.A., J.P., J.F.S., A.P., and M.T. are employees of Alzheon Inc. and hold stocks or stock options in Alzheon Inc. L.S. and X.Z. are employees of Wuxi Apptec that provides statistical contracting services to Alzheon Inc. K.B., E.M.R., J.H., and K.S. are scientific advisors to Alzheon Inc. and hold stocks or stock options in Alzheon Inc. N.D.P. is an investigator in the phase 2 and phase 3 studies of ALZ-801.
Figures
References
-
- Alzheimer’s Association Facts and Figures. 2024. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf.
-
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, et al. Prevalence of Apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. 10.1159/000334607. 10.1159/000334607 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

