Circulating small extracellular vesicles in Alzheimer's disease: a case-control study of neuro-inflammation and synaptic dysfunction
- PMID: 38902659
- PMCID: PMC11188177
- DOI: 10.1186/s12916-024-03475-z
Circulating small extracellular vesicles in Alzheimer's disease: a case-control study of neuro-inflammation and synaptic dysfunction
Abstract
Background: Alzheimer's disease (AD) is a neurodegenerative disease characterized by Aβ plaques and neurofibrillary tangles. Chronic inflammation and synaptic dysfunction lead to disease progression and cognitive decline. Small extracellular vesicles (sEVs) are implicated in AD progression by facilitating the spread of pathological proteins and inflammatory cytokines. This study investigates synaptic dysfunction and neuroinflammation protein markers in plasma-derived sEVs (PsEVs), their association with Amyloid-β and tau pathologies, and their correlation with AD progression.
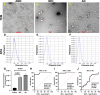
Methods: A total of 90 [AD = 35, mild cognitive impairment (MCI) = 25, and healthy age-matched controls (AMC) = 30] participants were recruited. PsEVs were isolated using a chemical precipitation method, and their morphology was characterized by transmission electron microscopy. Using nanoparticle tracking analysis, the size and concentration of PsEVs were determined. Antibody-based validation of PsEVs was done using CD63, CD81, TSG101, and L1CAM antibodies. Synaptic dysfunction and neuroinflammation were evaluated with synaptophysin, TNF-α, IL-1β, and GFAP antibodies. AD-specific markers, amyloid-β (1-42), and p-Tau were examined within PsEVs using Western blot and ELISA.
Results: Our findings reveal higher concentrations of PsEVs in AD and MCI compared to AMC (p < 0.0001). Amyloid-β (1-42) expression within PsEVs is significantly elevated in MCI and AD compared to AMC. We could also differentiate between the amyloid-β (1-42) expression in AD and MCI. Similarly, PsEVs-derived p-Tau exhibited elevated expression in MCI compared with AMC, which is further increased in AD. Synaptophysin exhibited downregulated expression in PsEVs from MCI to AD (p = 0.047) compared to AMC, whereas IL-1β, TNF-α, and GFAP showed increased expression in MCI and AD compared to AMC. The correlation between the neuropsychological tests and PsEVs-derived proteins (which included markers for synaptic integrity, neuroinflammation, and disease pathology) was also performed in our study. The increased number of PsEVs correlates with disease pathological markers, synaptic dysfunction, and neuroinflammation.
Conclusions: Elevated PsEVs, upregulated amyloid-β (1-42), and p-Tau expression show high diagnostic accuracy in AD. The downregulated synaptophysin expression and upregulated neuroinflammatory markers in AD and MCI patients suggest potential synaptic degeneration and neuroinflammation. These findings support the potential of PsEV-associated biomarkers for AD diagnosis and highlight synaptic dysfunction and neuroinflammation in disease progression.
Keywords: Alzheimer’s disease; Mild cognitive impairment; Neuroinflammation; Small extracellular vesicles; Synaptic dysfunction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Suescun J, Chandra S, Schiess MC. Chapter 13 - The role of neuroinflammation in neurodegenerative disorders. In: Actor JK, Smith KC, editors. Translational Inflammation. Academic Press; 2019. pp. 241–267.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

