Role of signaling pathways in age-related orthopedic diseases: focus on the fibroblast growth factor family
- PMID: 38902808
- PMCID: PMC11191355
- DOI: 10.1186/s40779-024-00544-5
Role of signaling pathways in age-related orthopedic diseases: focus on the fibroblast growth factor family
Abstract
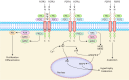
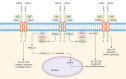
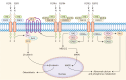
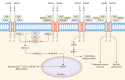
Fibroblast growth factor (FGF) signaling encompasses a multitude of functions, including regulation of cell proliferation, differentiation, morphogenesis, and patterning. FGFs and their receptors (FGFR) are crucial for adult tissue repair processes. Aberrant FGF signal transduction is associated with various pathological conditions such as cartilage damage, bone loss, muscle reduction, and other core pathological changes observed in orthopedic degenerative diseases like osteoarthritis (OA), intervertebral disc degeneration (IVDD), osteoporosis (OP), and sarcopenia. In OA and IVDD pathologies specifically, FGF1, FGF2, FGF8, FGF9, FGF18, FGF21, and FGF23 regulate the synthesis, catabolism, and ossification of cartilage tissue. Additionally, the dysregulation of FGFR expression (FGFR1 and FGFR3) promotes the pathological process of cartilage degradation. In OP and sarcopenia, endocrine-derived FGFs (FGF19, FGF21, and FGF23) modulate bone mineral synthesis and decomposition as well as muscle tissues. FGF2 and other FGFs also exert regulatory roles. A growing body of research has focused on understanding the implications of FGF signaling in orthopedic degeneration. Moreover, an increasing number of potential targets within the FGF signaling have been identified, such as FGF9, FGF18, and FGF23. However, it should be noted that most of these discoveries are still in the experimental stage, and further studies are needed before clinical application can be considered. Presently, this review aims to document the association between the FGF signaling pathway and the development and progression of orthopedic diseases. Besides, current therapeutic strategies targeting the FGF signaling pathway to prevent and treat orthopedic degeneration will be evaluated.
Keywords: Fibroblast growth factor (FGF); Fibroblast growth factor receptor (FGFR); Intervertebral disc degeneration (IVDD); Orthopedic degeneration; Osteoarthritis (OA); Osteoporosis (OP); Sarcopenia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFA0111900/National Key R&D Program of China
- 82072506/National Natural Science Foundation of China
- 92268115/National Natural Science Foundation of China
- 2020SK53709/Provincial Clinical Medical Technology Innovation Project of Hunan
- 2021RC3025/Science and Technology Innovation Program of Hunan Province
- 202204074879/the program of Health Commission of Hunan Province
- No.2022ZZTS0268/the Independent Exploration and Innovation Project for Postgraduate Students of Central South University
- No.CX20220350/the Hunan Provincial Innovation Foundation for Postgraduate
- 2021075/the Administration of Traditional Chinese Medicine of Hunan Province
- 2020CX045/Innovation-Driven Project of Central South University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

