Leptomeningeal metastases from solid tumors: A Society for Neuro-Oncology and American Society of Clinical Oncology consensus review on clinical management and future directions
- PMID: 38902944
- PMCID: PMC11449070
- DOI: 10.1093/neuonc/noae103
Leptomeningeal metastases from solid tumors: A Society for Neuro-Oncology and American Society of Clinical Oncology consensus review on clinical management and future directions
Abstract
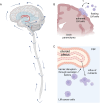
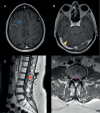
Leptomeningeal metastases (LM) are increasingly becoming recognized as a treatable, yet generally incurable, complication of advanced cancer. As modern cancer therapeutics have prolonged the lives of patients with metastatic cancer, specifically in patients with parenchymal brain metastases, treatment options, and clinical research protocols for patients with LM from solid tumors have similarly evolved to improve survival within specific populations. Recent expansions in clinical investigation, early diagnosis, and drug development have given rise to new unanswered questions. These include leptomeningeal metastasis biology and preferred animal modeling, epidemiology in the modern cancer population, ensuring validation and accessibility of newer leptomeningeal metastasis diagnostics, best clinical practices with multimodality treatment options, clinical trial design and standardization of response assessments, and avenues worthy of further research. An international group of multi-disciplinary experts in the research and management of LM, supported by the Society for Neuro-Oncology and American Society of Clinical Oncology, were assembled to reach a consensus opinion on these pressing topics and provide a roadmap for future directions. Our hope is that these recommendations will accelerate collaboration and progress in the field of LM and serve as a platform for further discussion and patient advocacy.
Keywords: consensus guideline; intrathecal therapy; leptomeningeal disease; leptomeningeal metastases; radiation therapy; systemic therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
E.P., E.Q.L., I.S., J.A.W., L.G., M.D., M.D.T., M.G., M.J.A., and S.C.: None. U.N.C.: receives consulting fees from Servier Pharmaceuticals. A.A.A.: receives research funding from Varian and NH TherAguix and consulting fees from Seagen and Novartis. T.A.B.: receives consulting fees from Biocartis. D.B.: receives consulting fees from Boehringer Ingelheim and Lilly Pharmaceuticals. P.K.B.: receives research support from Eli Lilly, AstraZeneca, Kazia, Genentech/Roche, G.S.K., and B.M.S., Kinnate, Mirati, and Merck; and consulting fees from Genentech-Roche, Axiom Healthcare Strategies, Dantari, Sintetica, M.P.M. Capital Advisors, Pfizer, Kazia, SK Life Sciences, Voyager Therapeutics, Elevate Bio, CraniUS, Medscape, Merk and Advise Connect Inspire. PF: receives consulting fees and research support from BMS, Pfizer, and Genentech. Isabella C. Glitza Oliva. PK: PKB has consulted for Angiochem, Merck, Genentech-Roche, Eli Lilly, Tesaro, ElevateBio, Dantari, Voyager Therapeutics, SK Life Sciences, Pfizer, ACI, Atavistik, Sintetica, Kazia, MPM, CraniUS, Axiom and InCephalo, serves on the Scientific Advisory Board for Kazia and CraniUS, has received grant/research support (to institution) from Merck, GSK, AstraZeneca, Kazia, Genentech-Roche, Eli Lilly, Mirati, BMS and Kinnate, and speaker’s honoraria from Merck, Genentech-Roche, Eli Lilly and Medscape. R.R.: receives consulting fees from Novocure, Servier, and CureVac; and research support from Bayer. M.W.: has received research support from Quercis and Versameb, and consulting fees from Bayer, Curevac, Medac, Neurosense, Novartis, Novocure, Orbus, Pfizer, Philogen, Roche, and Servier. J.W.: has received consulting fees from Intra-Cellular Therapies, Inc, GT Medical Technologies Inc, Juno Therapeutics, Novocure, and is on the advisory board for Bayer Inc., Astellas. J.T.Y.: received consulting fees from: Kazia Therapeutics, Plus Therapeutics, and AstraZeneca, and research support from Kazia Therapeutics, Biocept, and AstraZeneca. RJY: receives consulting fees from ICON plc, RadMD, NordicNeuroImaging, Olea Medical, and Turing Medical. P.Y.W.: receives Research Support from Astra Zeneca, Black Diamond, Bristol Meyers Squibb, Chimerix, Eli Lily, Erasca, Global Coalition For Adaptive Research, Kazia, MediciNova, Merck, Novartis, Quadriga, Servier, VBI Vaccines; and receives consulting fees from Anheart, Astra Zeneca, Black Diamond, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Kintara, Merck, Mundipharma, Novartis, Novocure, Prelude Therapeutics, Sagimet, Sapience, Servier, Symbio, Tango, Telix, VBI Vaccines. A.A.B.: receives consulting fees from Apellis Pharmaceuticals. J.R. and A.B. are inventors on the following provisional patent applications filed by MSKCC: International Patent Application No. PCT/US24/18343, US patent application no. 63/449,817, and 63/449,823.
Figures

References
-
- Wasserstrom WR, Glass JP, Posner JB.. Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer. 1982;49(4):759–772. - PubMed
-
- Posner JB. Neurologic complications of cancer. London: Oxford University Press; 1995.
-
- Shapiro WR, Posner JB, Ushio Y, Chemik NL, Young DF.. Treatment of meningeal neoplasms. Cancer Treat Rep. 1977;61(4):733–743. - PubMed
-
- Posner JB, Chernik NL.. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

