This is a preprint.
Regulation of angiogenesis by endocytic trafficking mediated by cytoplasmic dynein 1 light intermediate chain 1
- PMID: 38903077
- PMCID: PMC11188074
- DOI: 10.1101/2024.04.01.587559
Regulation of angiogenesis by endocytic trafficking mediated by cytoplasmic dynein 1 light intermediate chain 1
Update in
-
Angiogenesis is limited by LIC1-mediated lysosomal trafficking.Angiogenesis. 2024 Nov;27(4):943-962. doi: 10.1007/s10456-024-09951-7. Epub 2024 Oct 2. Angiogenesis. 2024. PMID: 39356418 Free PMC article.
Abstract
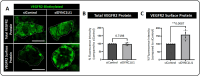
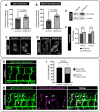
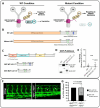
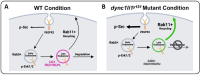
Dynein cytoplasmic 1 light intermediate chain 1 (LIC1, DYNC1LI1) is a core subunit of the dynein motor complex. The LIC1 subunit also interacts with various cargo adaptors to regulate Rab-mediated endosomal recycling and lysosomal degradation. Defects in this gene are predicted to alter dynein motor function, Rab binding capabilities, and cytoplasmic cargo trafficking. Here, we have identified a dync1li1 zebrafish mutant, harboring a premature stop codon at the exon 12/13 splice acceptor site, that displays increased angiogenesis. In vitro, LIC1-deficient human endothelial cells display increases in cell surface levels of the pro-angiogenic receptor VEGFR2, SRC phosphorylation, and Rab11-mediated endosomal recycling. In vivo, endothelial-specific expression of constitutively active Rab11a leads to excessive angiogenesis, similar to the dync1li1 mutants. Increased angiogenesis is also evident in zebrafish harboring mutations in rilpl1/2, the adaptor proteins that promote Rab docking to Lic1 to mediate lysosomal targeting. These findings suggest that LIC1 and the Rab-adaptor proteins RILPL1 and 2 restrict angiogenesis by promoting degradation of VEGFR2-containing recycling endosomes. Disruption of LIC1- and RILPL1/2-mediated lysosomal targeting increases Rab11-mediated recycling endosome activity, promoting excessive SRC signaling and angiogenesis.
Keywords: Lic1; Rilp1/2; angiogenesis; dynein motor; endosomes; lysosomes; zebrafish.
Conflict of interest statement
Competing Interests: The authors declare that they have no conflicts of interest.
Figures



References
-
- Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–94. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
