This is a preprint.
The UFMylation pathway is impaired in Alzheimer's disease
- PMID: 38903110
- PMCID: PMC11188091
- DOI: 10.1101/2024.05.24.595755
The UFMylation pathway is impaired in Alzheimer's disease
Update in
-
The UFMylation pathway is impaired in Alzheimer's disease.Mol Neurodegener. 2024 Dec 18;19(1):97. doi: 10.1186/s13024-024-00784-y. Mol Neurodegener. 2024. PMID: 39696466 Free PMC article.
Abstract
Background: Alzheimer's disease (AD) is characterized by the presence of neurofibrillary tangles made of hyperphosphorylated tau and senile plaques composed of beta-amyloid. These pathognomonic deposits have been implicated in the pathogenesis, although the molecular mechanisms and consequences remain undetermined. UFM1 is an important, but understudied ubiquitin-like protein that is covalently attached to substrates. This UFMylation has recently been identified as major modifier of tau aggregation upon seeding in experimental models. However, potential alterations of the UFM1 pathway in human AD brain have not been investigated yet.
Methods: Here we used frontal and temporal cortex samples from individuals with or without AD to measure the protein levels of the UFMylation pathway in human brain. We used multivariable regression analyses followed by Bonferroni correction for multiple testing to analyze associations of the UFMylation pathway with neuropathological characteristics, primary biochemical measurements of tau and additional biochemical markers from the same cases. We further studied associations of the UFMylation cascade with cellular stress pathways using Spearman correlations with bulk RNAseq expression data and functionally validated these interactions using gene-edited neurons that were generated by CRISPR-Cas9.
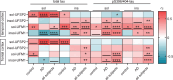
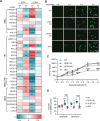
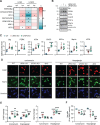
Results: Compared to controls, human AD brain had increased protein levels of UFM1. Our data further indicates that this increase mainly reflects conjugated UFM1 indicating hyperUFMylation in AD. UFMylation was strongly correlated with pathological tau in both AD-affected brain regions. In addition, we found that the levels of conjugated UFM1 were negatively correlated with soluble levels of the deUFMylation enzyme UFSP2. Functional analysis of UFM1 and/or UFSP2 knockout neurons revealed that the DNA damage response as well as the unfolded protein response are perturbed by changes in neuronal UFM1 signaling.
Conclusions: There are marked changes in the UFMylation pathway in human AD brain. These changes are significantly associated with pathological tau, supporting the idea that the UFMylation cascade might indeed act as a modifier of tau pathology in human brain. Our study further nominates UFSP2 as an attractive target to reduce the hyperUFMylation observed in AD brain but also underscores the critical need to identify risks and benefits of manipulating the UFMylation pathway as potential therapeutic avenue for AD.
Keywords: Alzheimer’s disease; UFM1; UFMylation; UFSP2; brain; tau.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests.
Figures


References
-
- Sasakawa H, Sakata E, Yamaguchi Y, Komatsu M, Tatsumi K, Kominami E, Tanaka K, Kato K: Solution structure and dynamics of Ufm1, a ubiquitin-fold modifier 1. Biochemical and Biophysical Research Communications 2006, 343:21–26. - PubMed
-
- Kang SH, Kim GR, Seong M, Baek SH, Seol JH, Bang OS, Ovaa H, Tatsumi K, Komatsu M, Tanaka K, Chung CH: Two novel ubiquitin-fold modifier 1 (Ufm1)-specific proteases, UfSP1 and UfSP2. J Biol Chem 2007, 282:5256–5262. - PubMed
-
- Mizushima T, Tatsumi K, Ozaki Y, Kawakami T, Suzuki A, Ogasahara K, Komatsu M, Kominami E, Tanaka K, Yamane T: Crystal structure of Ufc1, the Ufm1-conjugating enzyme. Biochemical and Biophysical Research Communications 2007, 362:1079–1084. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
