Re-pairing DNA: binding of a ruthenium phi complex to a double mismatch
- PMID: 38903237
- PMCID: PMC11186304
- DOI: 10.1039/d4sc01448k
Re-pairing DNA: binding of a ruthenium phi complex to a double mismatch
Erratum in
-
Correction: Re-pairing DNA: binding of a ruthenium phi complex to a double mismatch.Chem Sci. 2024 Sep 6;15(36):14988. doi: 10.1039/d4sc90174f. Online ahead of print. Chem Sci. 2024. PMID: 39246339 Free PMC article.
Abstract
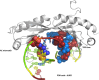
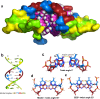
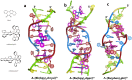
We report a crystal structure at atomic resolution (0.9 Å) of a ruthenium complex bound to a consecutive DNA double mismatch, which results in a TA basepair with flipped out thymine, together with the formation of an adenine bulge. The structure shows a form of metalloinsertion interaction of the Λ-[Ru(phen)2phi]2+ (phi = 9,10-phenanthrenediimine) complex at the bulge site. The metal complex interacts with the DNA via the major groove, where specific interactions between the adenines of the DNA and the phen ligands of the complex are formed. One Δ-[Ru(phen)2phi]2+ complex interacts via the minor groove, which shows sandwiching of its phi ligand between the phi ligands of the other two ruthenium complexes, and no interaction of its phen ligands with DNA. To our knowledge, this binding model represents a new form of metalloinsertion in showing major rather than minor groove insertion.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources

