Alkyl anchor-modified artificial viral capsid budding outside-to-inside and inside-to-outside giant vesicles
- PMID: 38903411
- PMCID: PMC11188953
- DOI: 10.1080/14686996.2024.2347191
Alkyl anchor-modified artificial viral capsid budding outside-to-inside and inside-to-outside giant vesicles
Abstract
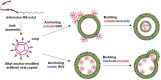
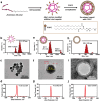
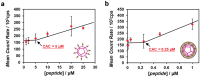
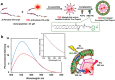
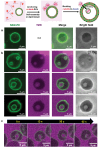
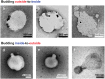
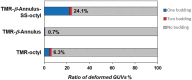
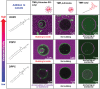
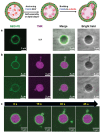
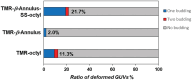
The budding of human immunodeficiency virus from an infected host cell is induced by the modification of structural proteins bearing long-chain fatty acids, followed by their anchoring to the cell membrane. Although many model budding systems using giant unilamellar vesicles (GUVs) induced by various stimuli have been developed, constructing an artificial viral budding system of GUVs using only synthesized molecules remains challenging. Herein, we report the construction of an artificial viral capsid budding system from a lipid bilayer of GUV. The C-terminus of the β-annulus peptide was modified using an octyl chain as an alkyl anchor via a disulfide bond. The self-assembly of the β-annulus peptide with an octyl chain formed an artificial viral capsid aggregate. The fluorescence imaging and transmission electron microscopy observations revealed that the addition of the tetramethylrhodamine (TMR)-labeled octyl chain-bearing β-annulus peptide to the outer aqueous phase of GUV induced the budding of the capsid-encapsulated daughter vesicle outside-to-inside the mother GUV. Conversely, the encapsulation of the TMR-labeled octyl chain-bearing β-annulus peptide in the inner aqueous phase of GUV induced the budding of the capsid-encapsulated daughter vesicle inside-to-outside the mother GUV. Contrarily, the addition of the TMR-labeled β-annulus peptide to GUV barely induced budding. It was demonstrated that the higher the membrane fluidity of GUV, the more likely budding would be induced by the addition of the alkyl anchor-modified artificial viral capsid. The simple virus-mimicking material developed in this study, which buds off through membrane anchoring, can provide physicochemical insights into the mechanisms of natural viral budding from cells.
Keywords: Artificial viral capsid; alkyl anchor; budding; giant vesicle; self-assembly; β-annulus peptide.
Plain language summary
Construction of an artificial viral budding system of GUVs using only synthesized molecules remains challenging. This study firstly demonstrates that budding outside-to-inside and inside-to-outside GUVs are induced by addition of alkyl anchor-modified artificial viral capsid.
© 2024 The Author(s). Published by National Institute for Materials Science in partnership with Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
A supramolecular system mimicking the infection process of an enveloped virus through membrane fusion.Sci Rep. 2023 Nov 15;13(1):19934. doi: 10.1038/s41598-023-47347-7. Sci Rep. 2023. PMID: 37968508 Free PMC article.
-
Strategy toward In-Cell Self-Assembly of an Artificial Viral Capsid from a Fluorescent Protein-Modified β-Annulus Peptide.ACS Synth Biol. 2024 Jun 21;13(6):1842-1850. doi: 10.1021/acssynbio.4c00135. Epub 2024 May 10. ACS Synth Biol. 2024. PMID: 38729919
-
Exploring Giant Unilamellar Vesicle Production for Artificial Cells - Current Challenges and Future Directions.Small Methods. 2023 Dec;7(12):e2300416. doi: 10.1002/smtd.202300416. Epub 2023 Jul 18. Small Methods. 2023. PMID: 37464561 Review.
-
Giant unilamellar vesicles - a perfect tool to visualize phase separation and lipid rafts in model systems.Acta Biochim Pol. 2009;56(1):33-9. Epub 2009 Mar 17. Acta Biochim Pol. 2009. PMID: 19287805 Review.
Cited by
-
Layer-by-Layer Nanoarchitectonics: A Method for Everything in Layered Structures.Materials (Basel). 2025 Feb 1;18(3):654. doi: 10.3390/ma18030654. Materials (Basel). 2025. PMID: 39942320 Free PMC article. Review.
-
Dynamic Flow-Assisted Nanoarchitectonics.ACS Appl Mater Interfaces. 2025 Apr 30;17(17):24778-24806. doi: 10.1021/acsami.5c03820. Epub 2025 Apr 21. ACS Appl Mater Interfaces. 2025. PMID: 40255047 Free PMC article. Review.
References
-
- Rheinemann L, Sundquist WI.. Virus. Budding. In: Bamford D, Zuckerman M, editors. Encyclopedia of virology. Cambridge (MA): Academic Press; 2021. p. 519–16. doi: 10.1016/B978-0-12-814515-9.00023-0 - DOI
-
- Grigorov B, Muriaux D, Argirova R, et al. New insights into human immunodeficiency virus—type 1 replication. Biotechnol Biotechnol Equip. 2005;19(1):3–15. doi: 10.1080/13102818.2005.10817147 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
