Acid-base reaction-based dispersive solid phase extraction of favipiravir using biotin from biological samples prior to capillary electrophoresis analysis
- PMID: 38903417
- PMCID: PMC11188667
- DOI: 10.1039/d3ra07356d
Acid-base reaction-based dispersive solid phase extraction of favipiravir using biotin from biological samples prior to capillary electrophoresis analysis
Abstract
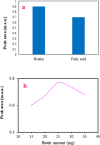
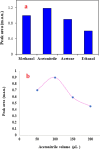
In this study, an acid-base reaction-based dispersive solid-phase extraction method was developed for the extraction of favipiravir from deionized water, plasma and urine samples prior to its determination using a capillary electrophoresis-diode array detector. The target analyte was extracted from the samples using biotin as a green adsorbent. To reach this goal, the pH of the solution was first adjusted to 9.0 (using borate buffer), and the ionic strength of the solution was enhanced by adding sodium chloride (2.5%, w/v). Thereafter, an appropriate amount of biotin was dissolved in the solution and a homogenous phase was obtained. By adding hydrochloric acid to the solution, an acid-base reaction occurs via protonation of biotin, which decreases its solubility. During this procedure, the analyte was adsorbed onto the tiny particles of the produced adsorbent dispersed into the solution. The resulting mixture was sonicated to facilitate the adsorption of the analyte onto the adsorbent surface. After the collection of biotin particles through centrifugation, the analyte was eluted using acetonitrile and then used in the determination stage. Under the optimal extraction conditions, the calibration curve was linear from 250 to 3000 ng mL-1 with a coefficient of determination of 0.9968. Low limit of detection, and quantification, good repeatability on the same day and different days (relative standard deviation ≤ 8.2%), and acceptable extraction recovery were accessed. The applicability of the method was examined by performing it on spiked plasma and urine samples, and its performance was verified.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures
Similar articles
-
Combination of dispersive solid phase extraction and deep eutectic solvent-based air-assisted liquid-liquid microextraction followed by gas chromatography-mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids.J Chromatogr A. 2018 Oct 12;1571:84-93. doi: 10.1016/j.chroma.2018.08.022. Epub 2018 Aug 10. J Chromatogr A. 2018. PMID: 30119972
-
[Preparation of sulfonic acid-functionalized polymeric ionic liquid-based magnetic adsorbent and its applications to diquat extraction].Se Pu. 2022 Oct;40(10):921-928. doi: 10.3724/SP.J.1123.2022.01027. Se Pu. 2022. PMID: 36222255 Free PMC article. Chinese.
-
UiO-66-based metal-organic framework for dispersive solid-phase extraction of vanillylmandelic acid from urine before analysis by capillary electrophoresis.RSC Adv. 2022 Oct 10;12(44):28728-28737. doi: 10.1039/d2ra02916b. eCollection 2022 Oct 4. RSC Adv. 2022. PMID: 36320520 Free PMC article.
-
H-beta zeolite-based dispersive solid-phase strategy for the multi-residue determination of pesticides.Anal Chim Acta. 2022 Sep 22;1227:340327. doi: 10.1016/j.aca.2022.340327. Epub 2022 Aug 29. Anal Chim Acta. 2022. PMID: 36089300
-
Development of a dispersive solid phase extraction method based on in situ formation of adsorbent followed by dispersive liquid-liquid microextraction for extraction of some pesticide residues in fruit juice samples.J Chromatogr A. 2020 Sep 13;1627:461398. doi: 10.1016/j.chroma.2020.461398. Epub 2020 Jul 8. J Chromatogr A. 2020. PMID: 32823103
References
-
- McKimm-Breschkin J. L. Jiang S. Hui D. S. Beigel J. H. Govorkova E. A. Lee N. Prevention and treatment of respiratory viral infections: Presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV antiviral group conference. Antiviral Res. 2018;149:118–142. - PMC - PubMed
-
- Du Y. X. Chen X. P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108:242–247. - PubMed
LinkOut - more resources
Full Text Sources

