Transmission from seed to seedling and elimination of alfalfa viruses
- PMID: 38903432
- PMCID: PMC11187482
- DOI: 10.3389/fpls.2024.1330219
Transmission from seed to seedling and elimination of alfalfa viruses
Abstract
Introduction: Viral diseases have become a vital factor limiting the development of the alfalfa (Medicago sativa) industry. Six viruses infecting alfalfa with a high incidence rate are Alfalfa mosaic virus (AMV), Medicago sativa alphapartitivirus 1 (MsAPV1), Medicago sativa alphapartitivirus 2 (MsAPV2), Medicago sativa deltapartitivirus 1 (MsDPV1), Medicago sativa amalgavirus 1 (MsAV1), and Cnidium vein yellowing virus 1 (CnVYV1). The purpose of this study was to develop preventive measures against these viruses by investigating their transmission through alfalfa seeds.
Methods: In this study, we investigated the transmission rate of alfalfa viruses from seed to seedling by PCR, determined the location of viruses in seed by dissecting seed embryos and seed coat, tracked the changes of viruses in seedlings, and finally discover effective elimination measures for alfalfa viruses from 16 measures.
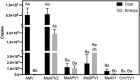
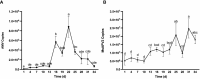
Results and discussion: Our results demonstrated that all these six viruses could be transmitted from alfalfa seeds to seedlings with the transmission rate ranging from 44.44% to 88.89%. For AMV, MsAPV2, and MsAV1, the viral load was significantly higher in the seed coats than in the seed embryos; however, it did not show significant differences between these two parts of the seeds for MsAPV1, MsDPV1, and CnVYV1. Dynamic accumulation analysis of AMV and MsAPV2 indicated that the viral load in plants increased continuously in the early growth stage, making it important to inactivate these viruses prior to their seed-to-seedling transmission. Sixteen treatments including physical, chemical, and combinations of physical and chemical measures were compared in terms of their elimination efficiency on AMV and MsAPV2 and impacts on seed germination. The results showed that soaking alfalfa seeds in sterile distilled water for 2h + 2% NaClO for 1h or 2% NaClO for 1h were more promisingly applicable because it could significantly reduce AMV and MsAPV2 particles in both seeds and seedlings. Our data revealed a route of virus transmission in alfalfa and shed light on the discovery of a highly efficient method for the management of alfalfa viral diseases.
Keywords: Medicago sativa alphapartitivirus 2 (MsAPV2); alfalfa mosaic virus (AMV); alfalfa viruses; transmission from seed to seedling; viruses elimination.
Copyright © 2024 Li, Shang, Luo, Wei, Zhao and Ban.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
RNA-seq reveals plant virus composition and diversity in alfalfa, thrips, and aphids in Beijing, China.Arch Virol. 2021 Jun;166(6):1711-1722. doi: 10.1007/s00705-021-05067-1. Epub 2021 Apr 17. Arch Virol. 2021. PMID: 33866416
-
Occurrence, Distribution, and Transmission of Alfalfa Viruses in China.Viruses. 2022 Jul 12;14(7):1519. doi: 10.3390/v14071519. Viruses. 2022. PMID: 35891498 Free PMC article.
-
Occurrence, Distribution, and Genetic Diversity of Alfalfa (Medicago sativa L.) Viruses in Four Major Alfalfa-Producing Provinces of China.Front Microbiol. 2022 Jan 13;12:771361. doi: 10.3389/fmicb.2021.771361. eCollection 2021. Front Microbiol. 2022. PMID: 35095791 Free PMC article.
-
Identification of novel RNA viruses in alfalfa (Medicago sativa): an Alphapartitivirus, a Deltapartitivirus, and a Marafivirus.Gene. 2018 Jan 5;638:7-12. doi: 10.1016/j.gene.2017.09.069. Epub 2017 Sep 30. Gene. 2018. PMID: 28974471
-
Australian Cool-Season Pulse Seed-Borne Virus Research: 1. Alfalfa and Cucumber Mosaic Viruses and Less Important Viruses.Viruses. 2024 Jan 18;16(1):144. doi: 10.3390/v16010144. Viruses. 2024. PMID: 38257844 Free PMC article. Review.
References
-
- Broadbent L. (1965). The epidemiology of tomato mosaic. Ann. Appl. Biol. 56, 177–205. doi: 10.1111/j.1744-7348.1965.tb01227.x - DOI
-
- Chang S., Puryear J., Cairney J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. doi: 10.1007/BF02670468 - DOI
LinkOut - more resources
Full Text Sources

