Real-world effectiveness of upadacitinib in Crohn's disease: a UK multicentre retrospective cohort study
- PMID: 38903490
- PMCID: PMC11187394
- DOI: 10.1136/flgastro-2024-102668
Real-world effectiveness of upadacitinib in Crohn's disease: a UK multicentre retrospective cohort study
Abstract
Background: Upadacitinib is a Janus kinase inhibitor, which has recently been approved for treating Crohn's disease. There are limited real-world studies on the outcomes of upadacitinib in Crohn's disease.
Objective: Our aim was to evaluate the outcomes of upadacitinib in a real-world Crohn's disease cohort.
Methods: We conducted a retrospective, multicentre, cohort study over a 2-year period across National Health Service (NHS) Lothian and Royal Devon University Healthcare NHS Foundation Trust. The primary outcome was treatment persistence at week 24. Secondary endpoints were corticosteroid-free clinical remission (Harvey-Bradshaw Index (HBI)<5) and biomarker remission (C-reactive protein (CRP)≤5 mg/L and faecal calprotectin (FCAL)<250 µg/g) at 12, 24 and 52 weeks. We recorded adverse events.
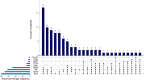
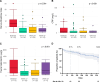
Results: 135 patients commenced upadacitinib as of the 1 January 2024, of which 93 patients with active Crohn's disease were included with a minimum of 12 weeks follow-up. The median follow-up time was 25 weeks (IQR 15-42 weeks). 82% of the cohort had exposure to at least two classes of advanced therapies, and 52% had exposure to at least three classes of advanced therapies. Treatment persistence was 87.1% at week 12, 81.7% at week 24 and 62.8% at week 52. Rates of clinical remission were 64% (42/66), 48% (22/46) and 38% (8/21) at weeks 12, 24 and 52, respectively. Significant reductions in HBI, CRP and FCAL were observed during follow-up. 14% (13/91) had a hospitalisation due to Crohn's disease. Adverse events occurred in 40% (37/93) of the cohort, of which 12% (11/93) were serious.
Conclusion: Upadacitinib was effective in a real-world, highly refractory, Crohn's disease cohort with good persistence.
Keywords: CROHN'S DISEASE; INFLAMMATORY BOWEL DISEASE.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BG has served as consultant to Galapagos and Abbvie and as speaker for Abbvie, Janssen, Takeda, Pfizer and Galapagos. NP has served as a speaker for Janssen, Takeda and Pfizer. JG has received grants from F. Hoffmann-La Roche AG, grants from Biogen, grants from Celltrion Healthcare, grants from Galapagos NV, nonfinancial support from Immundiagnostik, outside the submitted work. NAK has acted as a consultant to Amgen, Bristol Myers Squibb, Celltrion, Falk, Janssen, Pfizer, Pharmacosmos, Galapagos, Takeda and Tillotts, received speaking fees from Amgen, Celltrion, Falk, Janssen, Pharmacosmos, Galapagos, Takeda and Tillotts and travel support from AbbVie, Falk, Janssen and Pharmacosmos. His institution has received grants from AbbVie, Biogen, Celgene, Celltrion, Galapagos, MSD, Napp, Pfizer, Pharmacosmos, Roche and Takeda. TA reports grants and non-financial support from F. Hoffmann-La Roche AG, grants from Biogen, grants from Celltrion Healthcare, grants from Galapagos NV, non-financial support from Immundiagnostik, personal fees from Biogen, grants and personal fees from Celltrion Healthcare, personal fees and nonfinancial support from Immundiagnostik, personal fees from Takeda, personal fees from ARENA, personal fees from Gilead, personal fees from Adcock Ingram Healthcare, personal fees from Pfizer, personal fees from Genentech, nonfinancial support from Tillotts, outside the submitted work. CWL has acted as a consultant to Abbvie, Janssen, Takeda, Pfizer, Galapagos, Bristol Myers Squibb, B.I., Sandoz, Novartis, GSK, Gilead, ViforPharma, Dr Falk and Iterative Health; he has received speaking fees and travel support from Pfizer, Janssen, Abbvie, Galapagos, MSD, Takeda, Shire, Ferring, Hospira and Dr Falk.
Figures
References
-
- Padda IS, Bhatt R, Parmar M. Upadacitinib. In: StatPearls [Internet]. Treasure Island (FL), 2023. Available: https://www.ncbi.nlm.nih.gov/books/NBK572088/
-
- MHRA APPROVES Rinvoq[®]▼ (Upadacitinib) as first oral advanced therapy to treat adults with moderately to severely active Crohn’s disease. 2023. Available: https://news.cision.com/abbvie/r/mhra-approves-rinvoq---upadacitinib--as...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
