State-of-the-art diagnosis of autoimmune blistering diseases
- PMID: 38903493
- PMCID: PMC11187241
- DOI: 10.3389/fimmu.2024.1363032
State-of-the-art diagnosis of autoimmune blistering diseases
Abstract
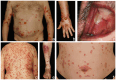
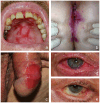
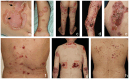
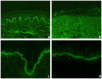
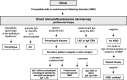
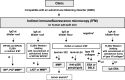
Autoimmune blistering disorders (AIBDs) are a heterogeneous group of approximately a dozen entities comprising pemphigus and pemphigoid disorders and dermatitis herpetiformis. The exact diagnosis of AIBDs is critical for both prognosis and treatment and is based on the clinical appearance combined with the detection of tissue-bound and circulating autoantibodies. While blisters and erosions on the skin and/or inspectable mucosal surfaces are typical, lesions may be highly variable with erythematous, urticarial, prurigo-like, or eczematous manifestations. While direct immunofluorescence microscopy (IFM) of a perilesional biopsy is still the diagnostic gold standard, the molecular identification of the major target antigens opened novel therapeutic avenues. At present, most AIBDs can be diagnosed by the detection of autoantigen-specific serum antibodies by enzyme-linked immunosorbent assay (ELISA) or indirect IFM when the clinical picture is known. This is achieved by easily available and highly specific and sensitive assays employing recombinant immunodominant fragments of the major target antigens, i.e., desmoglein 1 (for pemphigus foliaceus), desmoglein 3 (for pemphigus vulgaris), envoplakin (for paraneoplastic pemphigus), BP180/type XVII collagen (for bullous pemphigoid, pemphigoid gestationis, and mucous membrane pemphigoid), laminin 332 (for mucous membrane pemphigoid), laminin β4 (for anti-p200 pemphigoid), type VII collagen (for epidermolysis bullosa acquisita and mucous membrane pemphigoid), and transglutaminase 3 (for dermatitis herpetiformis). Indirect IFM on tissue substrates and in-house ELISA and immunoblot tests are required to detect autoantibodies in some AIBD patients including those with linear IgA disease. Here, a straightforward modern approach to diagnosing AIBDs is presented including diagnostic criteria according to national and international guidelines supplemented by long-term in-house expertise.
Keywords: autoantibody; autoimmune bullous disease; dermatitis herpetiformis; epidermolysis; immunofluorescence; linear IgA dermatosis; pemphigoid; pemphigus.
Copyright © 2024 van Beek, Holtsche, Atefi, Olbrich, Schmitz, Pruessmann, Vorobyev and Schmidt.
Conflict of interest statement
ES has a scientific cooperation with Euroimmun. NvB and ES have a pending patent together with Euroimmun. ES holds patents together with Euroimmun. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. ES declared that they was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

