Direct modulation of G protein-gated inwardly rectifying potassium (GIRK) channels
- PMID: 38903913
- PMCID: PMC11187414
- DOI: 10.3389/fphys.2024.1386645
Direct modulation of G protein-gated inwardly rectifying potassium (GIRK) channels
Abstract
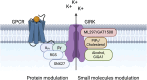
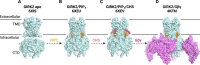
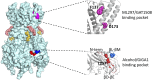
Ion channels play a pivotal role in regulating cellular excitability and signal transduction processes. Among the various ion channels, G-protein-coupled inwardly rectifying potassium (GIRK) channels serve as key mediators of neurotransmission and cellular responses to extracellular signals. GIRK channels are members of the larger family of inwardly-rectifying potassium (Kir) channels. Typically, GIRK channels are activated via the direct binding of G-protein βγ subunits upon the activation of G-protein-coupled receptors (GPCRs). GIRK channel activation requires the presence of the lipid signaling molecule, phosphatidylinositol 4,5-bisphosphate (PIP2). GIRK channels are also modulated by endogenous proteins and other molecules, including RGS proteins, cholesterol, and SNX27 as well as exogenous compounds, such as alcohol. In the last decade or so, several groups have developed novel drugs and small molecules, such as ML297, GAT1508 and GiGA1, that activate GIRK channels in a G-protein independent manner. Here, we aim to provide a comprehensive overview focusing on the direct modulation of GIRK channels by G-proteins, PIP2, cholesterol, and novel modulatory compounds. These studies offer valuable insights into the underlying molecular mechanisms of channel function, and have potential implications for both basic research and therapeutic development.
Keywords: G protein-gated inwardly rectifying potassium channel (GIRK); Kir3; PIP2; alcohol; cholesterol; small molecule modulator.
Copyright © 2024 Nguyen, Glaaser and Slesinger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anderson A., Masuho I., Fernandez de Velasco E. M., Nakano A., Lutz B., Martemyanov K. A., et al. (2020). GPCR-dependent biasing of GIRK channel signaling dynamics by RGS6 in mouse sinoatrial nodal cells. Proc. Natl. Acad. Sci. U. S. A. 117 (25), 14522–14531. 10.1073/pnas.2001270117 - DOI - PMC - PubMed
-
- Arora D., Hearing M., Haluk D. M., Mirkovic K., Fajardo-Serrano A., Wessendorf M. W., et al. (2011). Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J. Neurosci. Official J. Soc. Neurosci. 31 (34), 12251–12257. 10.1523/JNEUROSCI.0494-11.2011 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

