Gut microbes improve prognosis of Klebsiella pneumoniae pulmonary infection through the lung-gut axis
- PMID: 38903943
- PMCID: PMC11188585
- DOI: 10.3389/fcimb.2024.1392376
Gut microbes improve prognosis of Klebsiella pneumoniae pulmonary infection through the lung-gut axis
Abstract
Background: The gut microbiota plays a vital role in the development of sepsis and in protecting against pneumonia. Previous studies have demonstrated the existence of the gut-lung axis and the interaction between the gut and the lung, which is related to the prognosis of critically ill patients; however, most of these studies focused on chronic lung diseases and influenza virus infections. The purpose of this study was to investigate the effect of faecal microbiota transplantation (FMT) on Klebsiella pneumoniae-related pulmonary infection via the gut-lung axis and to compare the effects of FMT with those of traditional antibiotics to identify new therapeutic strategies.
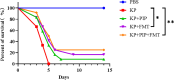
Methods: We divided the mice into six groups: the blank control (PBS), pneumonia-derived sepsis (KP), pneumonia-derived sepsis + antibiotic (KP + PIP), pneumonia-derived sepsis + faecal microbiota transplantation(KP + FMT), antibiotic treatment control (KP+PIP+PBS), and pneumonia-derived sepsis+ antibiotic + faecal microbiota transplantation (KP + PIP + FMT) groups to compare the survival of mice, lung injury, inflammation response, airway barrier function and the intestinal flora, metabolites and drug resistance genes in each group.
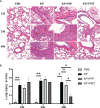
Results: Alterations in specific intestinal flora can occur in the gut of patients with pneumonia-derived sepsis caused by Klebsiella pneumoniae. Compared with those in the faecal microbiota transplantation group, the antibiotic treatment group had lower levels of proinflammatory factors and higher levels of anti-inflammatory factors but less amelioration of lung pathology and improvement of airway epithelial barrier function. Additionally, the increase in opportunistic pathogens and drug resistance-related genes in the gut of mice was accompanied by decreased production of favourable fatty acids such as acetic acid, propionic acid, butyric acid, decanoic acid, and secondary bile acids such as chenodeoxycholic acid 3-sulfate, isodeoxycholic acid, taurodeoxycholic acid, and 3-dehydrocholic acid; the levels of these metabolites were restored by faecal microbiota transplantation. Faecal microbiota transplantation after antibiotic treatment can gradually ameliorate gut microbiota disorder caused by antibiotic treatment and reduce the number of drug resistance genes induced by antibiotics.
Conclusion: In contrast to direct antibiotic treatment, faecal microbiota transplantation improves the prognosis of mice with pneumonia-derived sepsis caused by Klebsiella pneumoniae by improving the structure of the intestinal flora and increasing the level of beneficial metabolites, fatty acids and secondary bile acids, thereby reducing systemic inflammation, repairing the barrier function of alveolar epithelial cells, and alleviating pathological damage to the lungs. The combination of antibiotics with faecal microbiota transplantation significantly alleviates intestinal microbiota disorder, reduces the selection for drug resistance genes caused by antibiotics, and mitigates lung lesions; these effects are superior to those following antibiotic monotherapy.
Keywords: FMT; Klebsiella pneumoniae; antibiotheraphy; drug resistance gene; gut microbes; pulmonary infection.
Copyright © 2024 Tang, Chen, Yang, Zhang, Jin and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Protective effect of gut microbiota restored by fecal microbiota transplantation in a sepsis model in juvenile mice.Front Immunol. 2024 Oct 22;15:1451356. doi: 10.3389/fimmu.2024.1451356. eCollection 2024. Front Immunol. 2024. PMID: 39502702 Free PMC article.
-
Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances.Front Immunol. 2023 Jan 11;13:1063543. doi: 10.3389/fimmu.2022.1063543. eCollection 2022. Front Immunol. 2023. PMID: 36713461 Free PMC article.
-
The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia.Gut. 2016 Apr;65(4):575-83. doi: 10.1136/gutjnl-2015-309728. Epub 2015 Oct 28. Gut. 2016. PMID: 26511795 Free PMC article.
-
The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection.Therap Adv Gastroenterol. 2022 Nov 18;15:17562848221134396. doi: 10.1177/17562848221134396. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36425405 Free PMC article. Review.
-
Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: a systematic review and meta-analysis.J Hosp Infect. 2019 Jun;102(2):174-188. doi: 10.1016/j.jhin.2019.03.010. Epub 2019 Mar 26. J Hosp Infect. 2019. PMID: 30926290
Cited by
-
The Intriguing Connection Between the Gut and Lung Microbiomes.Pathogens. 2024 Nov 15;13(11):1005. doi: 10.3390/pathogens13111005. Pathogens. 2024. PMID: 39599558 Free PMC article.
-
Advances in gut-lung axis research: clinical perspectives on pneumonia prevention and treatment.Front Immunol. 2025 Apr 22;16:1576141. doi: 10.3389/fimmu.2025.1576141. eCollection 2025. Front Immunol. 2025. PMID: 40330490 Free PMC article. Review.
-
High-Calorie Diets Exacerbate Lipopolysaccharide-Induced Pneumonia by Promoting Propionate-Mediated Neutrophil Extracellular Traps.Nutrients. 2025 Jul 7;17(13):2242. doi: 10.3390/nu17132242. Nutrients. 2025. PMID: 40647346 Free PMC article.
-
DC-SIGN (CD209)-mediated interactions between bacteria, lung cancer tissues, and macrophages promote cancer metastasis.Infect Agent Cancer. 2025 Jun 21;20(1):40. doi: 10.1186/s13027-025-00667-x. Infect Agent Cancer. 2025. PMID: 40544275 Free PMC article.
References
-
- Ashurst J. V., Dawson A. (2023). Klebsiella Pneumonia. In: StatPearls. (Treasure Island (FL): StatPearls Publishing; ).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

