Comprehensive analysis of the endothelin system in the kidneys of mice, rats, and humans
- PMID: 38904098
- PMCID: PMC11249498
- DOI: 10.1042/BSR20240768
Comprehensive analysis of the endothelin system in the kidneys of mice, rats, and humans
Abstract
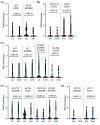
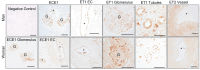
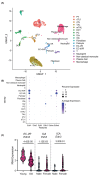
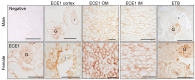
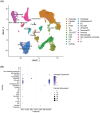
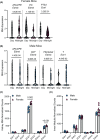
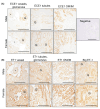
The intrarenal endothelin (ET) system is an established moderator of kidney physiology and mechanistic contributor to the pathophysiology and progression of chronic kidney disease in humans and rodents. The aim of the present study was to characterize ET system by combining single cell RNA sequencing (scRNA-seq) data with immunolocalization in human and rodent kidneys of both sexes. Using publicly available scRNA-seq data, we assessed sex and kidney disease status (human), age and sex (rats), and diurnal expression (mice) on the kidney ET system expression. In normal human biopsies of both sexes and in rodent kidney samples, the endothelin-converting enzyme-1 (ECE1) and ET-1 were prominent in the glomeruli and endothelium. These data agreed with the scRNA-seq data from these three species, with ECE1/Ece1 mRNA enriched in the endothelium. However, the EDN1/Edn1 gene (encodes ET-1) was rarely detected, even though it was immunolocalized within the kidneys, and plasma and urinary ET-1 excretion are easily measured. Within each species, there were some sex-specific differences. For example, in kidney biopsies from living donors, men had a greater glomerular endothelial cell endothelin receptor B (Ednrb) compared with women. In mice, females had greater kidney endothelial cell Ednrb than male mice. As commercially available antibodies did not work in all species, and RNA expression did not always correlate with protein levels, multiple approaches should be considered to maintain required rigor and reproducibility of the pre- and clinical studies evaluating the intrarenal ET system.
Keywords: Endothelin; RNA; chronic kidney disease; kidney; protein; sex.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Hypoxic regulation of EDN1, EDNRA, EDNRB, and ECE1 gene expressions in ERN1 knockdown U87 glioma cells.Endocr Regul. 2019 Oct 1;53(4):250-262. doi: 10.2478/enr-2019-0025. Endocr Regul. 2019. PMID: 31734650
-
ERN1 knockdown modifies the impact of glucose and glutamine deprivations on the expression of EDN1 and its receptors in glioma cells.Endocr Regul. 2021 May 21;55(2):72-82. doi: 10.2478/enr-2021-0009. Endocr Regul. 2021. PMID: 34020533
-
Differential regulation of the lung endothelin system by urban particulate matter and ozone.Toxicol Sci. 2005 Nov;88(1):103-13. doi: 10.1093/toxsci/kfi272. Epub 2005 Aug 4. Toxicol Sci. 2005. PMID: 16081523
-
Endothelin and endothelin receptors in the renal and cardiovascular systems.Life Sci. 2012 Oct 15;91(13-14):490-500. doi: 10.1016/j.lfs.2012.03.026. Epub 2012 Mar 28. Life Sci. 2012. PMID: 22480517 Review.
-
Endothelin@25 - new agonists, antagonists, inhibitors and emerging research frontiers: IUPHAR Review 12.Br J Pharmacol. 2014 Dec;171(24):5555-72. doi: 10.1111/bph.12874. Epub 2014 Nov 24. Br J Pharmacol. 2014. PMID: 25131455 Free PMC article. Review.
Cited by
-
The Impact of Mutant EDNRB on the Two-End Black Coat Color Phenotype in Chinese Local Pigs.Animals (Basel). 2025 Feb 7;15(4):478. doi: 10.3390/ani15040478. Animals (Basel). 2025. PMID: 40002960 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01DK126664/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01DK128001/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- UH3 DK114908/DK/NIDDK NIH HHS/United States
- U01 DK133090/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- UG3 DK114907/DK/NIDDK NIH HHS/United States
- U01 DK114933/DK/NIDDK NIH HHS/United States
- U01 DK114908/DK/NIDDK NIH HHS/United States
- UH3 DK114866/DK/NIDDK NIH HHS/United States
- U01 DK133081/DK/NIDDK NIH HHS/United States
- U01 DK114907/DK/NIDDK NIH HHS/United States
- UG3 DK114923/DK/NIDDK NIH HHS/United States
- U01 DK114920/DK/NIDDK NIH HHS/United States
- U24 DK114886/DK/NIDDK NIH HHS/United States
- U01 DK133766/DK/NIDDK NIH HHS/United States
- U01 DK114923/DK/NIDDK NIH HHS/United States
- U01 DK133113/DK/NIDDK NIH HHS/United States
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- R01 DK126664/DK/NIDDK NIH HHS/United States
- UG3 DK114908/DK/NIDDK NIH HHS/United States
- UG3 DK114866/DK/NIDDK NIH HHS/United States
- U01 DK114866/DK/NIDDK NIH HHS/United States
- UG3 DK114933/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- U01 DK133768/DK/NIDDK NIH HHS/United States
- U01 DK133092/DK/NIDDK NIH HHS/United States
- UG3 DK114920/DK/NIDDK NIH HHS/United States
- U01 DK133095/DK/NIDDK NIH HHS/United States
- K99/R00HL144817/National Heart, Lung, and Blood Institute (NHLBI)
- U01 DK133091/DK/NIDDK NIH HHS/United States
- U01 DK133093/DK/NIDDK NIH HHS/United States
- K99 HL144817/HL/NHLBI NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States
- R01 DK128001/DK/NIDDK NIH HHS/United States
- R00 HL144817/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

