In vitro activity of cefiderocol against European Enterobacterales, including isolates resistant to meropenem and recentβ-lactam/β-lactamase inhibitor combinations
- PMID: 38904361
- PMCID: PMC11302063
- DOI: 10.1128/spectrum.04181-23
In vitro activity of cefiderocol against European Enterobacterales, including isolates resistant to meropenem and recentβ-lactam/β-lactamase inhibitor combinations
Abstract
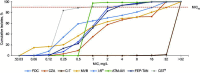
Carbapenem-resistant Enterobacterales represent a major health threat and have few approved therapeutic options. Enterobacterales isolates were collected from hospitalized inpatients from 49 sites in six European countries (1 January-31 December 2020) and underwent susceptibility testing to cefiderocol and β-lactam/β-lactamase inhibitor combinations. Meropenem-resistant (MIC >8 mg/L) and cefiderocol-susceptible isolates were analyzed by PCR, and cefiderocol-resistant isolates by whole-genome sequencing, to identify resistance mechanisms. Overall, 1,909 isolates (including 970 Klebsiella spp., 382 Escherichia coli, and 244 Enterobacter spp.) were collected, commonly from bloodstream infections (43.6%). Cefiderocol susceptibility was higher than approved β-lactam/β-lactamase inhibitor combinations and largely comparable to cefepime-taniborbactam and aztreonam-avibactam against all Enterobacterales (98.1% vs 78.1%-97.4% and 98.7%-99.1%, respectively) and Enterobacterales resistant to meropenem (n = 148, including 125 Klebsiella spp.; 87.8% vs 0%-71.6% and 93.2%-98.6%, respectively), β-lactam/β-lactamase inhibitor combinations (66.7%-92.1% vs 0%-88.1% and 66.7%-97.9%, respectively), and to both meropenem and β-lactam/β-lactamase inhibitor combinations (61.9%-65.9% vs 0%-20.5% and 76.2%-97.7%, respectively). Susceptibilities to approved and developmental β-lactam/β-lactamase inhibitor combinations against cefiderocol-resistant Enterobacterales (n = 37) were 10.8%-56.8% and 78.4%-94.6%, respectively. Most meropenem-resistant Enterobacterales harbored Klebsiella pneumoniae carbapenemase (110/148) genes, although metallo-β-lactamase (35/148) and oxacillinase (OXA) carbapenemase (6/148) genes were less common; cefiderocol susceptibility was retained in β-lactamase producers, other than NDM, AmpC, and non-carbapenemase OXA producers. Most cefiderocol-resistant Enterobacterales had multiple resistance mechanisms, including ≥1 iron uptake-related mutation (37/37), carbapenemase gene (33/37), and ftsI mutation (24/37). The susceptibility to cefiderocol was higher than approved β-lactam/β-lactamase inhibitor combinations against European Enterobacterales, including meropenem- and β-lactam/β-lactamase inhibitor combination-resistant isolates.
Importance: This study collected a notably large number of Enterobacterales isolates from Europe, including meropenem- and β-lactam/β-lactamase inhibitor combination-resistant isolates against which the in vitro activities of cefiderocol and developmental β-lactam/β-lactamase inhibitor combinations were directly compared for the first time. The MIC breakpoint for high-dose meropenem was used to define meropenem resistance, so isolates that would remain meropenem resistant with doses clinically available to patients were included in the data. Susceptibility to cefiderocol, as a single active compound, was high against Enterobacterales and was higher than or comparable to available β-lactam/β-lactamase inhibitor combinations. These results provide insights into the treatment options for infections due to Enterobacterales with resistant phenotypes. Early susceptibility testing of cefiderocol in parallel with β-lactam/β-lactamase inhibitor combinations will allow patients to receive the most appropriate treatment option(s) available in a timely manner. This is particularly important when options are more limited, such as against metallo-β-lactamase-producing Enterobacterales.
Keywords: Enterobacterales; Europe; Klebsiella pneumoniae; aztreonam-avibactam; cefepime-taniborbactam; cefiderocol; ceftazidime-avibactam; ceftolozane-tazobactam; imipenem-relebactam; meropenem; meropenem-vaborbactam; resistance.
Conflict of interest statement
A.S.H. is an employee of Maxel Consulting ApS, Jyllinge, Denmark, and is a contract employee of Shionogi B.V. R.C. declares honoraria from Pfizer, GSK, Shionogi, and Menarini and grants from Shionogi and MSD. T.N. declares a grant from Shionogi. I.M. is an employee of Antimicrobial Focus Ltd and a contract employee of IHMA. C.L. is an employee of Shionogi B.V. F.A., M.A., and S.G. have no relevant conflicts of interest to disclose.
Figures
References
-
- European Centre for Disease Prevention and Control . 2023. Antimicrobial resistance in the EU/EEA (EARS-Net) - annual epidemiological report for 2022. Stockholm. https://www.ecdc.europa.eu/sites/default/files/documents/AER-antimicrobi....
-
- Brolund A, Lagerqvist N, Byfors S, Struelens MJ, Monnet DL, Albiger B, Kohlenberg A, European Antimicrobial Resistance Genes Surveillance Network (EURGen-Net) capacity survey group . 2019. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Euro Surveill 24:1900123. doi:10.2807/1560-7917.ES.2019.24.9.1900123 - DOI - PMC - PubMed
-
- UK Health Security Agency . 2022. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR). London: Agency UHS. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
-
- European Centre for Disease Prevention and Control . 2019. Carbapenem-resistant Enterobacteriaceae – second update. Stockholm: ECDC. https://www.ecdc.europa.eu/sites/default/files/documents/carbapenem-resi....
-
- World Health Organization . 2017. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Geneva. https://apps.who.int/iris/handle/10665/311820.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

