Potential of cannabidiol as acne and acne scar treatment: novel insights into molecular pathways of pathophysiological factors
- PMID: 38904694
- PMCID: PMC11192675
- DOI: 10.1007/s00403-024-03131-9
Potential of cannabidiol as acne and acne scar treatment: novel insights into molecular pathways of pathophysiological factors
Abstract
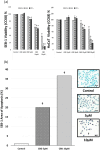
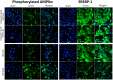
Cannabidiol (CBD), which is derived from hemp, is gaining recognition because of its anti-inflammatory and lipid-modulating properties that could be utilized to treat acne. We conducted experiments to quantitatively assess the effects of CBD on acne-related cellular pathways. SEB-1 sebocytes and HaCaT keratinocytes were exposed to various CBD concentrations. CBD exhibited a concentration-dependent impact on cell viability and notably reduced SEB-1 viability; furthermore, it induced apoptosis and a significant increase in the apoptotic area at higher concentrations. Additionally, CBD remarkably reduced pro-inflammatory cytokines, including CXCL8, IL-1α, and IL-1β. Additionally, it inhibited lipid synthesis by modulating the AMPK-SREBP-1 pathway and effectively reduced hyperkeratinization-related protein keratin 16. Simultaneously, CBD stimulated the synthesis of elastin, collagen 1, and collagen 3. These findings emphasize the potential of CBD for the management of acne because of its anti-inflammatory, apoptotic, and lipid-inhibitory effects. Notably, the modulation of the Akt/AMPK-SREBP-1 pathway revealed a novel and promising mechanism that could address the pathogenesis of acne.
Keywords: Acne; Cannabidiol; Inflammation; Keratinocytes; Lipid modulation; Sebocytes.
© 2024. The Author(s).
Conflict of interest statement
Jun Hyo Lee, Dong Hyo Kim, Ji Young Yoon and Dae Hun Suh declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Yoon Gyung Kwon, Geun-Hyeong Kim, and Byoung Jun Park are employees of Kolmar Korea, but they did not influence the interpretation of the experimental data.
Figures
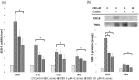


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

