Impact of Physical Exercise on Levodopa Therapy Across Parkinson's Disease Stages
- PMID: 38905055
- PMCID: PMC11307027
- DOI: 10.3233/JPD-230384
Impact of Physical Exercise on Levodopa Therapy Across Parkinson's Disease Stages
Abstract
Background: Levodopa is the gold standard of treatment in Parkinson's disease (PD). Its clinical effect changes as the disease progresses. Wearing off is a frequent first manifestation of motor fluctuations. Some patients with advanced PD report faster wearing off after physical exercise.
Objective: The aim was to assess if pharmacokinetics of levodopa is influenced by physical exercise in patients with different disease advancement.
Methods: 22 patients with PD (12 untreated with levodopa and 10 with motor fluctuations) and 7 healthy controls (HC) were included. Plasma samples were collected at 9 fixed timepoints following administration of levodopa/benserazide 200/50 mg for two days: rest day and standardized physical exercise day. Clinical assessment with Unified Parkinson Disease Rating Scale part III (UPDRS III) was performed in fixed timepoints. Liquid chromatography-tandem mass spectrometry was used to measure levodopa concentrations.
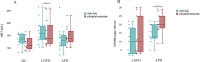
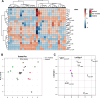
Results: No differences between the HC, levodopa naïve and advanced PD groups were observed regarding selected pharmacokinetic parameters. In advanced PD and HC no differences in pharmacokinetic parameters of levodopa with and without effort were observed. In levodopa naïve PD group higher mean residence time after rest than after exercise (168.9±48.3 min vs. 145.5±50.8 min; p = 0.026) was observed. In advanced PD group higher UPDRS III score (14.45±5.5 versus 20.9±6.1 points, p = 0.04) was observed after exercise.
Conclusions: The deterioration of motor status of advanced PD patients after physical effort is not reflected by changes in pharmacokinetics but rather mediated by central mechanisms.
Keywords: Levodopa; Parkinson’s disease; exercise; pharmacokinetics.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
Similar articles
-
Concurrent administration of donepezil HCl and levodopa/carbidopa in patients with Parkinson's disease: assessment of pharmacokinetic changes and safety following multiple oral doses.Br J Clin Pharmacol. 2004 Nov;58 Suppl 1(Suppl 1):41-9. doi: 10.1111/j.1365-2125.2004.01799.x. Br J Clin Pharmacol. 2004. PMID: 15496222 Free PMC article. Clinical Trial.
-
Pharmacokinetic-pharmacodynamic modeling of levodopa in patients with advanced Parkinson disease.Clin Neuropharmacol. 2010 May;33(3):135-41. doi: 10.1097/WNF.0b013e3181d47849. Clin Neuropharmacol. 2010. PMID: 20216409 Clinical Trial.
-
Effect of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor fluctuations in patients with Parkinson's disease.Eur J Neurol. 2015 May;22(5):815-25, e56. doi: 10.1111/ene.12666. Epub 2015 Feb 4. Eur J Neurol. 2015. PMID: 25649051 Clinical Trial.
-
Comparison of pramipexole and levodopa/benserazide combination therapy versus levodopa/benserazide monotherapy in the treatment of Parkinson's disease: a systematic review and meta-analysis.Naunyn Schmiedebergs Arch Pharmacol. 2021 Sep;394(9):1893-1905. doi: 10.1007/s00210-021-02089-z. Epub 2021 May 7. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33959780
-
Pharmacokinetics of Rytary®, An Extended-Release Capsule Formulation of Carbidopa-Levodopa.Clin Pharmacokinet. 2017 Sep;56(9):999-1014. doi: 10.1007/s40262-017-0511-y. Clin Pharmacokinet. 2017. PMID: 28236251 Free PMC article. Review.
Cited by
-
Enhanced catalytic stability for L-Dopa synthesis through cross-linking of Al₂O₃ nanocrystals with Bacillus subtilis tyrosine hydroxylase.World J Microbiol Biotechnol. 2025 Jul 28;41(8):282. doi: 10.1007/s11274-025-04492-7. World J Microbiol Biotechnol. 2025. PMID: 40719842 Free PMC article.
-
Safety of Immersive Virtual Reality for the Management of Parkinson's Disease.Sensors (Basel). 2024 Dec 22;24(24):8188. doi: 10.3390/s24248188. Sensors (Basel). 2024. PMID: 39771922 Free PMC article. Clinical Trial.
References
-
- Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276, 374–379. - PubMed
-
- Nutt JG (2008) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23, S580–S584. - PubMed
-
- Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F, Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease (STRIDE-PD) Investigators (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28, 1064–1071. - PubMed
-
- Fox SH, Katzenschlager R, Lim SY, Barton B, De Bie RM, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33, 1248–1266. - PubMed
-
- Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: A review. JAMA 323, 548–560. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

