Serotonergic Regulation of Synaptic Dopamine Levels Mitigates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease
- PMID: 38905058
- PMCID: PMC11307072
- DOI: 10.3233/JPD-240080
Serotonergic Regulation of Synaptic Dopamine Levels Mitigates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease
Abstract
Background: The serotonin (5-HT) system can manipulate the processing of exogenous L-DOPA in the DA-denervated striatum, resulting in the modulation of L-DOPA-induced dyskinesia (LID).
Objective: To characterize the effects of the serotonin precursor 5-hydroxy-tryptophan (5-HTP) or the serotonin transporter (SERT) inhibitor, Citalopram on L-DOPA-induced behavior, neurochemical signals, and underlying protein expressions in an animal model of Parkinson's disease.
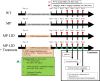
Methods: MitoPark (MP) mice at 20 weeks of age, subjected to a 14-day administration of L-DOPA/Carbidopa, displayed dyskinesia, referred to as LID. Subsequent investigations explored the effects of 5-HT-modifying agents, such as 5-HTP and Citalopram, on abnormal involuntary movements (AIMs), locomotor activity, neurochemical signals, serotonin transporter activity, and protein expression in the DA-denervated striatum of LID MP mice.
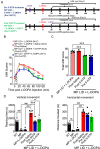
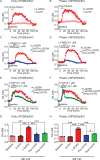
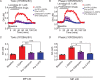
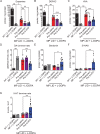
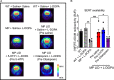
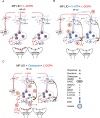
Results: 5-HTP exhibited duration-dependent suppressive effects on developing and established LID, especially related to abnormal limb movements observed in L-DOPA-primed MP mice. However, Citalopram, predominantly suppressed abnormal axial movement induced by L-DOPA in LID MP mice. We demonstrated that 5-HTP could decrease L-DOPA-upregulation of DA turnover rates while concurrently upregulating 5-HT metabolism. Additionally, 5-HTP was shown to reduce the expressions of p-ERK and p-DARPP-32 in the striatum of LID MP mice. The effect of Citalopram in alleviating LID development may be attributed to downregulation of SERT activity in the dorsal striatum of LID MP mice.
Conclusions: While both single injection of 5-HTP and Citalopram effectively mitigated the development of LID, the difference in mitigation of AIM subtypes may be linked to the unique effects of these two serotonergic agents on L-DOPA-derived DA and 5-HT metabolism.
Keywords: 5-HTP; Citalopram; L-DOPA-induced dyskinesia; Parkinson’s disease; Serotonin system; dopamine.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures



References
-
- Bogetofte H, Alamyar A, Blaabjerg M, Meyer M (2020) Levodopa therapy for Parkinson’s disease: History, current status and perspectives. CNS Neurol Disord 19, 572–583. - PubMed
-
- Santini E (2009) Molecular basis of L-DOPA-induced dyskinesia: Studies on striatal signaling, Karolinska Institutet, Sweden.
-
- Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut P-O, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R (2015) Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 132, 96–168. - PubMed
-
- de la Fuente-Fernández R, Sossi V, Huang Z, Furtado S, Lu J-Q, Calne DB, Ruth TJ, Stoessl AJ (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127, 2747–2754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

