Effect of Fenofibrate on Progression of Diabetic Retinopathy
- PMID: 38905569
- PMCID: PMC7616293
- DOI: 10.1056/EVIDoa2400179
Effect of Fenofibrate on Progression of Diabetic Retinopathy
Abstract
Background: Findings from cardiovascular outcome trials suggest that fenofibrate therapy may reduce the progression of diabetic retinopathy.
Methods: We recruited and followed adults with nonreferable diabetic retinopathy or maculopathy using the national Diabetic Eye Screening (DES) program in Scotland. We randomly assigned participants to receive 145-mg fenofibrate tablets or placebo (taken daily or, in those with impaired renal function, on alternate days). The primary outcome was a composite of developing referable diabetic retinopathy or maculopathy (based on Scotland's DES grading scheme) or treatment (intravitreal injection, retinal laser, vitrectomy) for retinopathy or maculopathy.
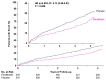
Results: A total of 1151 participants were randomly assigned to treatment. During a median of 4.0 years, progression to referable diabetic retinopathy or maculopathy, or treatment thereof, occurred in 131 (22.7%) of 576 participants in the fenofibrate group and 168 (29.2%) of 575 in the placebo group (hazard ratio, 0.73; 95% confidence interval [CI], 0.58 to 0.91; P=0.006). In the fenofibrate group compared with the placebo group, the frequencies for any progression of retinopathy or maculopathy were 185 (32.1%) vs. 231 (40.2%); hazard ratio, 0.74; 95% CI, 0.61 to 0.90 and for the development of macular edema were 22 (3.8%) vs. 43 (7.5%); hazard ratio, 0.50; 95% CI, 0.30 to 0.84. Seventeen (3.0%) participants assigned fenofibrate and 28 (4.9%) assigned placebo were given treatment for retinopathy (hazard ratio, 0.58; 95% CI, 0.31 to 1.06). There was no effect on visual function, quality of life, or visual acuity. Trial-averaged estimated glomerular filtration rate was 7.9 (95% CI, 6.8 to 9.1) ml/min/1.73 m2 lower in participants in the fenofibrate group compared with the placebo group. Serious adverse events occurred in 208 (36.1%) participants allocated fenofibrate and 204 (35.5%) participants allocated placebo.
Conclusions: Fenofibrate reduced progression of diabetic retinopathy compared with placebo among participants with early retinal changes. (Funded by the National Institute for Health and Care Research; ClinicalTrials.gov number, NCT03439345; ISRCTN number, ISRCTN15073006.).
Figures


Comment in
-
Fenofibrate Shows Promise in Slowing Diabetic Retinopathy Progression.NEJM Evid. 2024 Aug;3(8):EVIDe2400205. doi: 10.1056/EVIDe2400205. Epub 2024 Jul 23. NEJM Evid. 2024. PMID: 39041872 No abstract available.
References
-
- Global Burden of Disease 2019 Blindness and Vision Impairment Collaborators and Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e60.
-
- WHO Regional Office for Europe. Diabetic retinopathy screening: a short guide. Increase effectiveness, maximize benefits and minimize harm. 2020. [last accessed 22 April 2024]. at: https://www.who.int/europe/publications/i/item/9789289055321.
-
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–93. - PubMed
-
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
