Novel selective inhibitors of macropinocytosis-dependent growth in pancreatic ductal carcinoma
- PMID: 38906021
- PMCID: PMC11287759
- DOI: 10.1016/j.biopha.2024.116991
Novel selective inhibitors of macropinocytosis-dependent growth in pancreatic ductal carcinoma
Abstract
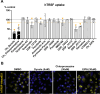
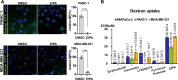
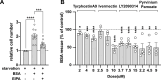
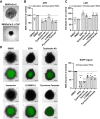
Macropinocytosis is a cellular process that enables cells to engulf extracellular material, such as nutrients, growth factors, and even whole cells. It is involved in several physiological functions as well as pathological conditions. In cancer cells, macropinocytosis plays a crucial role in promoting tumor growth and survival under nutrient-limited conditions. In particular KRAS mutations have been identified as main drivers of macropinocytosis in pancreatic, breast, and non-small cell lung cancers. We performed a high-content screening to identify inhibitors of macropinocytosis in pancreatic ductal adenocarcinoma (PDAC)-derived cells, aiming to prevent nutrient scavenging of PDAC tumors. The screening campaign was conducted in a well-known pancreatic KRAS-mutated cell line (MIAPaCa-2) cultured under nutrient deprivation and using FITC-dextran to precisely quantify macropinocytosis. We assembled a collection of 3584 small molecules, including drugs approved by the Food and Drug Administration (FDA), drug-like molecules against molecular targets, kinase-targeted compounds, and molecules designed to hamper protein-protein interactions. We identified 28 molecules that inhibited macropinocytosis, with potency ranging from 0.4 to 29.9 μM (EC50). A few of them interfered with other endocytic pathways, while 11 compounds did not and were therefore considered specific "bona fide" macropinocytosis inhibitors and further characterized. Four compounds (Ivermectin, Tyrphostin A9, LY2090314, and Pyrvinium Pamoate) selectively hampered nutrient scavenging in KRAS-mutated cancer cells. Their ability to impair albumin-dependent proliferation was replicated both in different 2D cell culture systems and 3D organotypic models. These findings provide a new set of compounds specifically targeting macropinocytosis, which could have therapeutic applications in cancer and infectious diseases.
Keywords: Drug repurposing; Drug screening; FDA-approved drugs; Ivermectin (PubChem CID: 6321424); LY2090314 (PubChem CID: 10029385); Macropinocytosis; Pancreatic ductal adenocarcinoma (PDAC); Pyrvinium Pamoate (PubChem CID: 54680693); Tyrphostin A9 (PubChem CID: 5614).
Copyright © 2024 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no competing interests.
Figures






References
-
- Kerr M.C., Teasdale R.D. Defining macropinocytosis. Traffic. 2009;10(4):364–371. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

