PJA1-mediated suppression of pyroptosis as a driver of docetaxel resistance in nasopharyngeal carcinoma
- PMID: 38906860
- PMCID: PMC11192944
- DOI: 10.1038/s41467-024-49675-2
PJA1-mediated suppression of pyroptosis as a driver of docetaxel resistance in nasopharyngeal carcinoma
Abstract
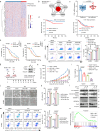
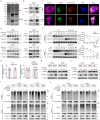
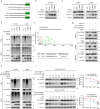
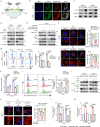
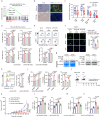
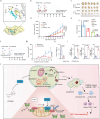
Chemoresistance is a main reason for treatment failure in patients with nasopharyngeal carcinoma, but the exact regulatory mechanism underlying chemoresistance in nasopharyngeal carcinoma remains to be elucidated. Here, we identify PJA1 as a key E3 ubiquitin ligase involved in nasopharyngeal carcinoma chemoresistance that is highly expressed in nasopharyngeal carcinoma patients with nonresponse to docetaxel-cisplatin-5-fluorouracil induction chemotherapy. We find that PJA1 facilitates docetaxel resistance by inhibiting GSDME-mediated pyroptosis in nasopharyngeal carcinoma cells. Mechanistically, PJA1 promotes the degradation of the mitochondrial protein PGAM5 by increasing its K48-linked ubiquitination at K88, which further facilitates DRP1 phosphorylation at S637 and reduced mitochondrial reactive oxygen species production, resulting in suppression of GSDME-mediated pyroptosis and the antitumour immune response. PGAM5 knockdown fully restores the docetaxel sensitization effect of PJA1 knockdown. Moreover, pharmacological targeting of PJA1 with the small molecule inhibitor RTA402 enhances the docetaxel sensitivity of nasopharyngeal carcinoma in vitro and in vivo. Clinically, high PJA1 expression indicates inferior survival and poor clinical efficacy of TPF IC in nasopharyngeal carcinoma patients. Our study emphasizes the essential role of E3 ligases in regulating chemoresistance and provides therapeutic strategies for nasopharyngeal carcinoma based on targeting the ubiquitin-proteasome system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sun Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi: 10.1016/S1470-2045(16)30410-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

