MiR-503-5p alleviates peripheral neuropathy-induced neuropathic pain in T2DM mice by regulating SEPT9 to inhibit astrocyte activation
- PMID: 38906977
- PMCID: PMC11192719
- DOI: 10.1038/s41598-024-65096-z
MiR-503-5p alleviates peripheral neuropathy-induced neuropathic pain in T2DM mice by regulating SEPT9 to inhibit astrocyte activation
Abstract
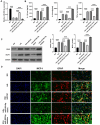
Diabetic peripheral neuropathy (DPN) is a common complication of type 2 diabetes mellitus (T2DM) that causes peripheral and autonomic nervous system dysfunction. Dysregulation of miRNAs plays a crucial role in DPN development. However, the role of miR-503-5p in DPN remains unknown. Herein, T2DM mice (db/db) were used as a DPN model in vivo, and astrocytes isolated from db/db mice were induced with high glucose levels as a DPN model in vitro. MiR-503-5p expression was analyzed using qRT-PCR. GFAP, MCP-1, and SEPT9 protein levels were analyzed using western blotting and immunofluorescence. Luciferase assays were performed to investigate the interaction between miR-503-5p and SEPT9. We found that miR-503-5p expression decreased in the spinal cord of DPN model mice and astrocytes treated with high glucose (HG). The db/db mice displayed higher body weight and blood glucose, lower mechanical withdrawal threshold and thermal withdrawal latency, and higher GFAP and MCP-1 protein levels than db/m mice. However, tail vein injection of agomiR-503-5p remarkably reversed these parameters, whereas antigomiR-503-5p enhanced them. HG markedly facilitated GFAP and MCP-1 protein expression in astrocytes, whereas miR-503-5p mimic or inhibitor transfection markedly blocked or elevated GFAP and MCP-1 protein expression, respectively, in astrocytes with HG. SEPT9 was a target of miR-503-5p. In addition, SEPT9 protein levels were found to be elevated in db/db mice and astrocytes treated with HG. Treatment with agomiR-503-5p and miR-503-5p mimic was able to reduce SEPT9 protein levels, whereas treatment with antigomiR-503-5p and miR-503-5p inhibitor led to inhibition of the protein. Furthermore, SEPT9 overexpression suppressed the depressing effect of miR-503-5p overexpression in astrocytes subjected to HG doses. In conclusion, miR-503-5p was found to alleviate peripheral neuropathy-induced neuropathic pain in T2DM mice by regulating SEPT9 expression.
Keywords: Astrocytes; Diabetic peripheral neuropathy; SEPT9; T2DM mice; miR-503-5p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Jeyam A, McGurnaghan SJ, Blackbourn LAK, McKnight JM, Green F, Collier A, McKeigue PM, Colhoun HM. Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: a population-representative study from Scotland. Diabetes Care. 2020;43(4):734–742. doi: 10.2337/dc19-1582. - DOI - PubMed
-
- Kasimu A, Apizi X, Talifujiang D, Ma X, Fang L, Zhou X. miR-125a-5p in astrocytes attenuates peripheral neuropathy in type 2 diabetic mice through targeting TRAF6. Endocrinol. Diabetes Nutr. (Engl Ed) 2022;69(1):43–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

