Pharmacovigilance in Pregnancy Studies, Exposures and Outcomes Ascertainment, and Findings from Low- and Middle-Income Countries: A Scoping Review
- PMID: 38907172
- PMCID: PMC11399196
- DOI: 10.1007/s40264-024-01445-1
Pharmacovigilance in Pregnancy Studies, Exposures and Outcomes Ascertainment, and Findings from Low- and Middle-Income Countries: A Scoping Review
Abstract
Introduction: Pharmacovigilance (PV), or the ongoing safety monitoring after a medication has been licensed, plays a crucial role in pregnancy, as clinical trials often exclude pregnant people. It is important to understand how pregnancy PV projects operate in low- and middle-income countries (LMICs), where there is a disproportionate lack of PV data yet a high burden of adverse pregnancy outcomes. We conducted a scoping review to assess how exposures and outcomes were measured in recently published pregnancy PV projects in LMICs.
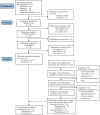
Methods: We utilized a search string, secondary review, and team knowledge to review publications focusing on therapeutic or vaccine exposures among pregnant people in LMICs. We screened abstracts for relevance before conducting a full text review, and documented measurements of exposures and outcomes (categorized as maternal, birth, or neonatal/infant) among other factors, including study topic, setting, and design, comparator groups, and funding sources.
Results: We identified 31 PV publications spanning at least 24 LMICs, all focusing on therapeutics or vaccines for infectious diseases, including HIV (n = 17), tuberculosis (TB; n = 9), malaria (n = 7), pertussis, tetanus, and diphtheria (n = 1), and influenza (n = 3). As for outcomes, n = 15, n = 31, and n = 20 of the publications covered maternal, birth, and neonatal/infant outcomes, respectively. Among HIV-specific publications, the primary exposure-outcome relationship of focus was exposure to maternal antiretroviral therapy and adverse outcomes. For TB-specific publications, the main exposures of interest were second-line drug-resistant TB and isoniazid-based prevention therapeutics for pregnant people living with HIV. For malaria-specific publications, the primary exposure-outcome relationship of interest was antimalarial medication exposure during pregnancy and adverse outcomes. Among vaccine-focused publications, the exposure was assessed during a specific time during pregnancy, with an overall interest in vaccine safety and/or efficacy. The study settings were frequently from Africa, designs varied from cohort or cross-sectional studies to clinical trials, and funding sources were largely from high-income countries.
Conclusion: The published pregnancy PV projects were largely centered in Africa and concerned with infectious diseases. This may reflect the disease burden in LMICs but also funding priorities from high-income countries. As the prevalence of non-communicable diseases increases in LMICs, PV projects will have to broaden their scope. Birth and neonatal/infant outcomes were most reported, with fewer reporting on maternal outcomes and none on longer-term child outcomes; additionally, heterogeneity existed in definitions and ascertainment of specific measures. Notably, almost all projects covered a single therapeutic exposure, missing an opportunity to leverage their projects to cover additional exposures, add scientific rigor, create uniformity across health services, and bolster existing health systems. For many publications, the timing of exposure, specifically by trimester, was crucial to maternal and neonatal safety. While currently published pregnancy PV literature offer insights into the PV landscape in LMICs, further work is needed to standardize definitions and measurements, integrate PV projects across health services, and establish longer-term monitoring.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflicts of interests to declare that are relevant to the content of this article.
Figures
References
-
- Regulation and Prequalification [Internet]. [cited 2023 Nov 2]. https://www.who.int/teams/regulation-prequalification/regulation-and-saf.... Accessed 2 Nov 2023.
-
- Division of Pediatric and Maternal Health - Clinical Trials in Pregnant Women | FDA [Internet]. [cited 2023 Oct 24]. https://www.fda.gov/drugs/development-resources/division-pediatric-and-m.... Accessed 24 Oct 2023.
Publication types
MeSH terms
Grants and funding
- R61 HD103093/HD/NICHD NIH HHS/United States
- R61HD103093/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- K23HD109056/National Institute of Child Health and Human Development
- U01AI069911/National Institute of Allergy and Infectious Diseases
- K23 HD109056/HD/NICHD NIH HHS/United States
- K23 HD105495/HD/NICHD NIH HHS/United States
- R01 HD080465/HD/NICHD NIH HHS/United States
- K23 MH116808/MH/NIMH NIH HHS/United States
- K23HD105495/National Institute of Child Health and Human Development
- R01HD080465/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- K23MH116808/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U01 AI069911/AI/NIAID NIH HHS/United States
- U01A1069924/National Institute of Allergy and Infectious Diseases
- U01 AI069924/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials