A Comprehensive Study of Light Quality Acclimation in Synechocystis Sp. PCC 6803
- PMID: 38907526
- PMCID: PMC11369814
- DOI: 10.1093/pcp/pcae062
A Comprehensive Study of Light Quality Acclimation in Synechocystis Sp. PCC 6803
Erratum in
-
Correction to: A Comprehensive Study of Light Quality Acclimation in Synechocystis Sp. PCC 6803.Plant Cell Physiol. 2025 Jul 24;66(6):971. doi: 10.1093/pcp/pcaf049. Plant Cell Physiol. 2025. PMID: 40400404 Free PMC article. No abstract available.
Abstract
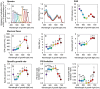
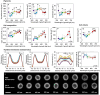
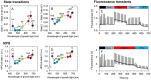
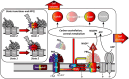
Cyanobacteria play a key role in primary production in both oceans and fresh waters and hold great potential for sustainable production of a large number of commodities. During their life, cyanobacteria cells need to acclimate to a multitude of challenges, including shifts in intensity and quality of incident light. Despite our increasing understanding of metabolic regulation under various light regimes, detailed insight into fitness advantages and limitations under shifting light quality remains underexplored. Here, we study photo-physiological acclimation in the cyanobacterium Synechocystis sp. PCC 6803 throughout the photosynthetically active radiation (PAR) range. Using light emitting diodes (LEDs) with qualitatively different narrow spectra, we describe wavelength dependence of light capture, electron transport and energy transduction to main cellular pools. In addition, we describe processes that fine-tune light capture, such as state transitions, or the efficiency of energy transfer from phycobilisomes to photosystems (PS). We show that growth was the most limited under blue light due to inefficient light harvesting, and that many cellular processes are tightly linked to the redox state of the plastoquinone (PQ) pool, which was the most reduced under red light. The PSI-to-PSII ratio was low under blue photons, however, it was not the main growth-limiting factor, since it was even more reduced under violet and near far-red lights, where Synechocystis grew faster compared to blue light. Our results provide insight into the spectral dependence of phototrophic growth and can provide the foundation for future studies of molecular mechanisms underlying light acclimation in cyanobacteria, leading to light optimization in controlled cultivations.
Keywords: Cyanobacteria; Light harvesting; Light quality; Photomorphogenesis; Photosynthesis; State transitions.
© The Author(s) 2024. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures


References
-
- Ajlani G. and Vernotte C. (1998) Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 37: 577–580. - PubMed
-
- Bernát G., Steinbach G., Kaňa R., Govindjee, Misra A.N., Prašil O. and Prašil O. (2018) On the origin of the slow M–T chlorophyll a fluorescence decline in cyanobacteria: interplay of short-term light-responses. Photosynth. Res. 136: 183–198. - PubMed
-
- Bernát G., Zavřel T., Kotabová E., Kovács L., Steinbach G., Vörös L., et al. (2021) Photomorphogenesis in the picocyanobacterium Cyanobium gracile includes increased phycobilisome abundance under blue light, phycobilisome decoupling under near far-red light, and wavelength-specific photoprotective strategies. Front Plant Sci. 12: 1–16. - PMC - PubMed
-
- Calzadilla P.I. and Kirilovsky D. (2020) Revisiting cyanobacterial state transitions. Photochem. Photobiol. Sci. 19: 585–603. - PubMed
MeSH terms
Substances
Grants and funding
- CZ.02.1.01/0.0/0.0/16_026/0008413 LM2018123 LUAUS24131/Ministerstvo Školství, Mládeže a Telovýchovy
- K 140351 RRF-2.3.1-21-2022-00014/Nemzeti Kutatási, Fejlesztési és Innovaciós Alap
- CZ.02.1.01/0.0/0.0/16_026/0008413 LM2018123 LUAUS24131/Ministerstvo Školství, Mládeže a Telovýchovy
- K 140351 RRF-2.3.1-21-2022-00014/Nemzeti Kutatási, Fejlesztési és Innovaciós Alap
LinkOut - more resources
Full Text Sources

