Ferroptosis inhibitor improves outcome after early and delayed treatment in mild spinal cord injury
- PMID: 38907771
- PMCID: PMC11193702
- DOI: 10.1007/s00401-024-02758-2
Ferroptosis inhibitor improves outcome after early and delayed treatment in mild spinal cord injury
Abstract
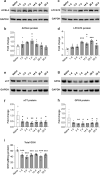
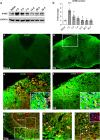
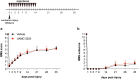
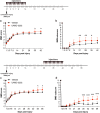
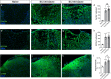
We show that redox active iron can induce a regulated form of non-apoptotic cell death and tissue damage called ferroptosis that can contribute to secondary damage and functional loss in the acute and chronic periods after spinal cord injury (SCI) in young, adult, female mice. Phagocytosis of red blood cells at sites of hemorrhage is the main source of iron derived from hemoglobin after SCI. Expression of hemeoxygenase-1 that induces release of iron from heme, is increased in spinal cord macrophages 7 days after injury. While iron is stored safely in ferritin in the injured spinal cord, it can, however, be released by NCOA4-mediated shuttling of ferritin to autophagosomes for degradation (ferritinophagy). This leads to the release of redox active iron that can cause free radical damage. Expression of NCOA4 is increased after SCI, mainly in macrophages. Increase in the ratio of redox active ferrous (Fe2+) to ferric iron (Fe3+) is also detected after SCI by capillary electrophoresis inductively coupled mass spectrometry. These changes are accompanied by other hallmarks of ferroptosis, i.e., deficiency in various elements of the antioxidant glutathione (GSH) pathway. We also detect increases in enzymes that repair membrane lipids (ACSL4 and LPCAT3) and thus promote on-going ferroptosis. These changes are associated with increased levels of 4-hydroxynonenal (4-HNE), a toxic lipid peroxidation product. Mice with mild SCI (30 kdyne force) treated with the ferroptosis inhibitor (UAMC-3203-HCL) either early or delayed times after injury showed improvement in locomotor recovery and secondary damage. Cerebrospinal fluid and serum samples from human SCI cases show evidence of increased iron storage (ferritin), and other iron related molecules, and reduction in GSH. Collectively, these data suggest that ferroptosis contributes to secondary damage after SCI and highlights the possible use of ferroptosis inhibitors to treat SCI.
Keywords: Antioxidants; Ferritin; Iron toxicity; Lipid peroxidation; NCOA4; Spinal cord injury.
© 2024. The Author(s).
Conflict of interest statement
None.
Figures


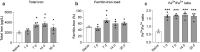


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

