Late-Onset Reactions after Hyaluronic Acid Dermal Fillers: A Consensus Recommendation on Etiology, Prevention and Management
- PMID: 38907876
- PMCID: PMC11265052
- DOI: 10.1007/s13555-024-01202-3
Late-Onset Reactions after Hyaluronic Acid Dermal Fillers: A Consensus Recommendation on Etiology, Prevention and Management
Abstract
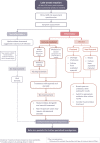
Hyaluronic acid (HA) dermal fillers, generally considered low-risk, can lead to rare late-onset reactions (LORs) manifesting between 3 and 4 months postinjection, occasionally even as early as 24 h postinjection. The Complication Assessment and Risk Evaluation (CARE) board was established to review these reactions. In this publication, the authors aims to explore the etiological hypotheses underlying LORs, associated risk factors, prevention, and management approaches suggested by the CARE board. The CARE board identified three etiological hypotheses contributing to LORs. Firstly, the physicochemical structure of the filler, particularly low molecular weight HA, which may trigger an immune response. Secondly, infection, potentially introduced during injection or by dormant biofilm activation. Lastly, an imbalance in the host immune system, caused by factors like autoimmune diseases or viral infections, may lead to extended foreign body reactions, delayed type IV hypersensitivity, or adjuvant-based reactions. Based on these hypotheses, the board categorized various risk factors as patient-related (e.g., recent dental treatment, current medical status, active autoimmune disease), product-related (e.g., molecular weight), and procedure-related (e.g., aseptic technique and trauma). To reduce the risk of LORs, the CARE board recommends diligent patient selection, including comprehensive medical history assessment and informed consent. Practitioners should maintain an effective aseptic technique, and choose an appropriate product and injection depth for the anatomical location. Post-procedure, patients should receive education on proper filler care. Management of LORs depends on the suspected etiology, and the CARE board has proposed an algorithm to determine the most appropriate treatment. Hyaluronidase is recommended for noninflammatory reactions in the absence of active infection, while watchful waiting and/or steroid treatment may be preferred for inflammatory reactions. Hyaluronidase is not recommended as a first-line treatment for infections, which require drainage, bacterial culture, and antibiotic treatment. However, the board emphasizes the need for individualized evaluation and treatment in all cases.
Keywords: Delayed onset reactions; Expert opinion; Infection; Inflammation; Treatment algorithm.
© 2024. The Author(s).
Conflict of interest statement
Professor Barańska-Rybak serves as a medical consultant for Teoxane SA in Poland. Dr Lajo-Plaza serves as a medical consultant for Teoxane SA in Spain. Dr Walker acts as a global key opinion leader for Teoxane SA and medical education consultant for Revance Therapeutics Inc. Dr Alizadeh serves as a medical consultant for Teoxane SA and for Relife (Menarini Group company, Italy) in Switzerland.
Figures
References
-
- Surgeons ASoP (2022) Plastic Surgery Statistics Report 2022 [Available from: https://www.plasticsurgery.org/documents/News/Statistics/2022/plastic-su..., Assessed in November 2023
-
- Heydenrych I, Kapoor KM, De Boulle K, Goodman G, Swift A, Kumar N, Rahman E. A 10-point plan for avoiding hyaluronic acid dermal filler-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018;11:603–11. 10.2147/CCID.S180904 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources