Population Pharmacokinetics of Inotuzumab Ozogamicin in Pediatric Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia: Results of Study ITCC-059
- PMID: 38907948
- PMCID: PMC11271359
- DOI: 10.1007/s40262-024-01386-z
Population Pharmacokinetics of Inotuzumab Ozogamicin in Pediatric Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia: Results of Study ITCC-059
Abstract
Background and objective: Inotuzumab ozogamicin is an antibody-drug conjugate approved for treating relapsed/refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in adults. Pediatric pharmacokinetic data of inotuzumab ozogamicin are lacking. This study is the first to examine the population pharmacokinetics of inotuzumab ozogamicin in pediatric patients with relapsed/refractory BCP-ALL.
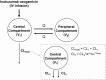
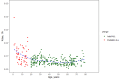
Methods: From 531 adult patients with B-cell non-Hodgkin's lymphoma, 234 adult patients with BCP-ALL, and 53 pediatric patients with BCP-ALL, 8924 inotuzumab ozogamicin serum concentrations were analyzed using non-linear mixed-effects modeling. A published adult inotuzumab ozogamicin population-pharmacokinetic model, a two-compartment model with linear and time-dependent clearance, was adapted to describe the pediatric data.
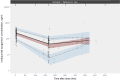
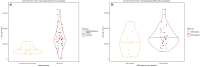
Results: Modifications in this analysis, compared to the published adult model, included: (i) re-estimating pharmacokinetic parameters and covariate effects; (ii) modifying covariate representation; and (iii) introducing relevant pediatric covariate effects (age on the decay coefficient of time-dependent clearance and ALL effect (disease type and/or different bioanalytical analysis methods) on initial values of time-dependent clearance). For patients with relapsed/refractory BCP-ALL, increasing age was associated with a decreasing decay coefficient of time-dependent clearance, reflecting that the target-mediated drug clearance declines more rapidly in children. In pediatric BCP-ALL, the median [interquartile range] cumulative area under the concentration-time curve was significantly higher among responders (n = 42) versus non-responders (n = 10) at the end of the first cycle (26.1 [18.9-35.0] vs 10.1 [9.19-16.1], × 103 ng*h/mL, p < 0.001). From simulations performed at the recommended pediatric phase II dose, inotuzumab ozogamicin exposure reached a similar level as observed in responding pediatric trial participants.
Conclusions: The pharmacokinetic profile of inotuzumab ozogamicin in pediatric patients with relapsed/refractory BCP-ALL was well described in this study. No dose adjustment is required clinically for pediatric patients with BCP-ALL based on the simulated inotuzumab ozogamicin exposure at the recommended pediatric phase II dose, promising efficacy and acceptable tolerability.
© 2024. The Author(s).
Conflict of interest statement
Susana Rives reports honoraria and/or travel support from Novartis, Servier, Celgene/Bristol Myers Squibb, Kite/ Gilead, Pfizer, and Amgen. Susana Rives reports being part of a Date and Safety Monitoring Board (DMSB) in a clinical trial sponsored by Novartis and of a data monitoring committee in a clinical trial sponsored by Autolus. Alwin D.R. Huitema is an Editorial Board member of
Figures




References
-
- World Health Organization. (2021). CureAll framework: WHO global initiative for childhood cancer: increasing access, advancing quality, saving lives. World Health Organization. n.d. https://iris.who.int/handle/10665/347370.
-
- Möricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–84. 10.1038/LEU.2009.257. 10.1038/LEU.2009.257 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

