Enhanced Prostate-specific Membrane Antigen Targeting by Precision Control of DNA Scaffolded Nanoparticle Ligand Presentation
- PMID: 38907991
- PMCID: PMC11223598
- DOI: 10.1021/acsnano.4c01640
Enhanced Prostate-specific Membrane Antigen Targeting by Precision Control of DNA Scaffolded Nanoparticle Ligand Presentation
Abstract
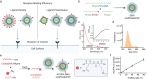
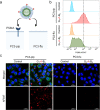
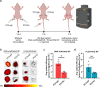
Targeted nanoparticles have been extensively explored for their ability to deliver their payload to a selective cell population while reducing off-target side effects. The design of actively targeted nanoparticles requires the grafting of a ligand that specifically binds to a highly expressed receptor on the surface of the targeted cell population. Optimizing the interactions between the targeting ligand and the receptor can maximize the cellular uptake of the nanoparticles and subsequently improve their activity. Here, we evaluated how the density and presentation of the targeting ligands dictate the cellular uptake of nanoparticles. To do so, we used a DNA-scaffolded PLGA nanoparticle system to achieve efficient and tunable ligand conjugation. A prostate-specific membrane antigen (PSMA) expressing a prostate cancer cell line was used as a model. The density and presentation of PSMA targeting ligand ACUPA were precisely tuned on the DNA-scaffolded nanoparticle surface, and their impact on cellular uptake was evaluated. It was found that matching the ligand density with the cell receptor density achieved the maximum cellular uptake and specificity. Furthermore, DNA hybridization-mediated targeting chain rigidity of the DNA-scaffolded nanoparticle offered ∼3 times higher cellular uptake compared to the ACUPA-terminated PLGA nanoparticle. Our findings also indicated a ∼ 3.7-fold reduction in the cellular uptake for the DNA hybridization of the non-targeting chain. We showed that nanoparticle uptake is energy-dependent and follows a clathrin-mediated pathway. Finally, we validated the preferential tumor targeting of the nanoparticles in a bilateral tumor xenograft model. Our results provide a rational guideline for designing actively targeted nanoparticles and highlight the application of DNA-scaffolded nanoparticles as an efficient active targeting platform.
Keywords: DNA; cell uptake; ligand density; nanoparticles; surface chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

