The long Pentraxin PTX3 serves as an early predictive biomarker of co-infections in COVID-19
- PMID: 38908098
- PMCID: PMC11245991
- DOI: 10.1016/j.ebiom.2024.105213
The long Pentraxin PTX3 serves as an early predictive biomarker of co-infections in COVID-19
Erratum in
-
The long Pentraxin PTX3 serves as an early predictive biomarker of co-infections in COVID-19.EBioMedicine. 2024 Oct;108:105372. doi: 10.1016/j.ebiom.2024.105372. Epub 2024 Sep 18. EBioMedicine. 2024. PMID: 39299004 Free PMC article. No abstract available.
Abstract
Background: COVID-19 clinical course is highly variable and secondary infections contribute to COVID-19 complexity. Early detection of secondary infections is clinically relevant for patient outcome. Procalcitonin (PCT) and C-reactive protein (CRP) are the most used biomarkers of infections. Pentraxin 3 (PTX3) is an acute phase protein with promising performance as early biomarker in infections. In patients with COVID-19, PTX3 plasma concentrations at hospital admission are independent predictor of poor outcome. In this study, we assessed whether PTX3 contributes to early identification of co-infections during the course of COVID-19.
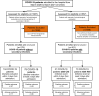
Methods: We analyzed PTX3 levels in patients affected by COVID-19 with (n = 101) or without (n = 179) community or hospital-acquired fungal or bacterial secondary infections (CAIs or HAIs).
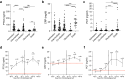
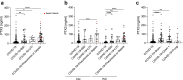
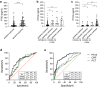
Findings: PTX3 plasma concentrations at diagnosis of CAI or HAI were significantly higher than those in patients without secondary infections. Compared to PCT and CRP, the increase of PTX3 plasma levels was associated with the highest hazard ratio for CAIs and HAIs (aHR 11.68 and 24.90). In multivariable Cox regression analysis, PTX3 was also the most significant predictor of 28-days mortality or intensive care unit admission of patients with potential co-infections, faring more pronounced than CRP and PCT.
Interpretation: PTX3 is a promising predictive biomarker for early identification and risk stratification of patients with COVID-19 and co-infections.
Funding: Dolce & Gabbana fashion house donation; Ministero della Salute for COVID-19; EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT) and MUR PNRR Italian network of excellence for advanced diagnosis (Project no. PNC-E3-2022-23683266 PNC-HLS-DA); EU MSCA (project CORVOS 860044).
Keywords: Biomarker; COVID-19; Community-acquired infections; Hospital-acquired infections; PTX3.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests A.M., B.B. and C.G. are inventors of a patent (EP20182181) on PTX3 and obtain royalties on related reagents. The other authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

