The neurophysiological brain-fingerprint of Parkinson's disease
- PMID: 38908100
- PMCID: PMC11253223
- DOI: 10.1016/j.ebiom.2024.105201
The neurophysiological brain-fingerprint of Parkinson's disease
Abstract
Background: Research in healthy young adults shows that characteristic patterns of brain activity define individual "brain-fingerprints" that are unique to each person. However, variability in these brain-fingerprints increases in individuals with neurological conditions, challenging the clinical relevance and potential impact of the approach. Our study shows that brain-fingerprints derived from neurophysiological brain activity are associated with pathophysiological and clinical traits of individual patients with Parkinson's disease (PD).
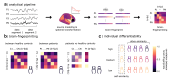
Methods: We created brain-fingerprints from task-free brain activity recorded through magnetoencephalography in 79 PD patients and compared them with those from two independent samples of age-matched healthy controls (N = 424 total). We decomposed brain activity into arrhythmic and rhythmic components, defining distinct brain-fingerprints for each type from recording durations of up to 4 min and as short as 30 s.
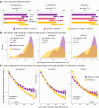
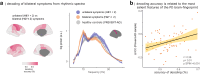
Findings: The arrhythmic spectral components of cortical activity in patients with Parkinson's disease are more variable over short periods, challenging the definition of a reliable brain-fingerprint. However, by isolating the rhythmic components of cortical activity, we derived brain-fingerprints that distinguished between patients and healthy controls with about 90% accuracy. The most prominent cortical features of the resulting Parkinson's brain-fingerprint are mapped to polyrhythmic activity in unimodal sensorimotor regions. Leveraging these features, we also demonstrate that Parkinson's symptom laterality can be decoded directly from cortical neurophysiological activity. Furthermore, our study reveals that the cortical topography of the Parkinson's brain-fingerprint aligns with that of neurotransmitter systems affected by the disease's pathophysiology.
Interpretation: The increased moment-to-moment variability of arrhythmic brain-fingerprints challenges patient differentiation and explains previously published results. We outline patient-specific rhythmic brain signaling features that provide insights into both the neurophysiological signature and symptom laterality of Parkinson's disease. Thus, the proposed definition of a rhythmic brain-fingerprint of Parkinson's disease may contribute to novel, refined approaches to patient stratification. Symmetrically, we discuss how rhythmic brain-fingerprints may contribute to the improved identification and testing of therapeutic neurostimulation targets.
Funding: Data collection and sharing for this project was provided by the Quebec Parkinson Network (QPN), the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer's Disease (PREVENT-AD; release 6.0) program, the Cambridge Centre for Aging Neuroscience (Cam-CAN), and the Open MEG Archives (OMEGA). The QPN is funded by a grant from Fonds de Recherche du Québec - Santé (FRQS). PREVENT-AD was launched in 2011 as a $13.5 million, 7-year public-private partnership using funds provided by McGill University, the FRQS, an unrestricted research grant from Pfizer Canada, the Levesque Foundation, the Douglas Hospital Research Centre and Foundation, the Government of Canada, and the Canada Fund for Innovation. The Brainstorm project is supported by funding to SB from the NIH (R01-EB026299-05). Further funding to SB for this study included a Discovery grant from the Natural Sciences and Engineering Research Council of Canada of Canada (436355-13), and the CIHR Canada research Chair in Neural Dynamics of Brain Systems (CRC-2017-00311).
Keywords: Arrhythmic brain activity; Brain-fingerprinting; Magnetoencephalography; Movement disorders; Neural dynamics; Oscillations; Parkinson’s disease.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no competing conflicts of interest. The listed funding sources in the Acknowledgements did not play any role in the writing of the manuscript or the decision to submit this manuscript for publication.
Figures


Update of
-
The neurophysiological brain-fingerprint of Parkinson's disease.medRxiv [Preprint]. 2023 Dec 1:2023.02.03.23285441. doi: 10.1101/2023.02.03.23285441. medRxiv. 2023. Update in: EBioMedicine. 2024 Jul;105:105201. doi: 10.1016/j.ebiom.2024.105201. PMID: 36798232 Free PMC article. Updated. Preprint.
References
-
- Stoffers D., Bosboom J.L.W., Deijen J.B., Wolters E.C., Berendse H.W., Stam C.J. Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain. 2007;130:1847–1860. - PubMed
-
- Stoffers D., Bosboom J.L.W., Wolters E. Ch., Stam C.J., Berendse H.W. Dopaminergic modulation of cortico-cortical functional connectivity in Parkinson’s disease: an MEG study. Exp Neurol. 2008;213:191–195. - PubMed
-
- Bosboom J.L.W., Stoffers D., Stam C.J., et al. Resting state oscillatory brain dynamics in Parkinson’s disease: an MEG study. Clin Neurophysiol. 2006;117:2521–2531. - PubMed
-
- Jubault T., Gagnon J., Karama S., et al. Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage. 2011;55:462–467. - PubMed
-
- Hanganu A., Bedetti C., Degroot C., Mejia-Constain B., Lafontaine A. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain J Neurol. 2014;137:1120–1129. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

