Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation
- PMID: 38908436
- PMCID: PMC11881887
- DOI: 10.1016/j.jhep.2024.06.016
Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation
Abstract
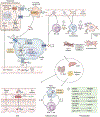
Chronic liver disease leads to hepatocellular injury that triggers a pro-inflammatory state in several parenchymal and non-parenchymal hepatic cell types, ultimately resulting in liver fibrosis, cirrhosis, portal hypertension and liver failure. Thus, an improved understanding of inflammasomes - as key molecular drivers of liver injury - may result in the development of novel diagnostic or prognostic biomarkers and effective therapeutics. In liver disease, innate immune cells respond to hepatic insults by activating cell-intrinsic inflammasomes via toll-like receptors and NF-κB, and by releasing pro-inflammatory cytokines (such as IL-1β, IL-18, TNF-α and IL-6). Subsequently, cells of the adaptive immune system are recruited to fuel hepatic inflammation and hepatic parenchymal cells may undergo gasdermin D-mediated programmed cell death, termed pyroptosis. With liver disease progression, there is a shift towards a type 2 inflammatory response, which promotes tissue repair but also fibrogenesis. Inflammasome activation may also occur at extrahepatic sites, such as the white adipose tissue in MASH (metabolic dysfunction-associated steatohepatitis). In end-stage liver disease, flares of inflammation (e.g., in severe alcohol-related hepatitis) that spark on a dysfunctional immune system, contribute to inflammasome-mediated liver injury and potentially result in organ dysfunction/failure, as seen in ACLF (acute-on-chronic liver failure). This review provides an overview of current concepts regarding inflammasome activation in liver disease progression, with a focus on related biomarkers and therapeutic approaches that are being developed for patients with liver disease.
Keywords: Kupffer cell; alcohol-related liver disease; cirrhosis; hepatic stellate cell; inflammasome; liver fibrosis; macrophage; metabolic dysfunction-associated steatotic liver disease; toll-like receptor, nod-like receptor.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
VT is funded by the Christian-Doppler Research Association and Boehringer-Ingelheim RCV GmbH & Co KG (CD10271603); Romanian Ministry of Education (HG 118/2023); Romanian Ministry of Research, Innovation and Digitalization (PNRR/2022/C9/MCID/I8). GS received consults for Durect, Merck, Pfizer, Labcorp, Surrozen, Intercept, Evive, Cyta Therapeutics and Pandion Therapeutics; has stock options in Glympse Bio, Satellite and Ventyx Bio. WM received consulting/advisory board fees from Novo Nordisk and Pfizer. TR received grant support from Abbvie, Boehringer-Ingelheim, Gilead, Gore, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, and Siemens; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Abbvie, Boehringer-Ingelheim, Gilead and Roche.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

