Surgery or endovascular therapy for patients with chronic limb-threatening ischemia requiring infrapopliteal interventions
- PMID: 38908805
- PMCID: PMC11633720
- DOI: 10.1016/j.jvs.2024.05.049
Surgery or endovascular therapy for patients with chronic limb-threatening ischemia requiring infrapopliteal interventions
Abstract
Objective: The recent publication of randomized trials comparing open bypass surgery to endovascular therapy in patients with chronic limb-threatening ischemia, namely, Best Endovascular vs Best Surgical Therapy in Patients with Critical Limb Ischemia (BEST-CLI) and Bypass versus Angioplasty in Severe Ischaemia of the Leg-2 (BASIL-2), has resulted in potentially contradictory findings. The trials differed significantly with respect to anatomical disease patterns and primary end points. We performed an analysis of patients in BEST-CLI with significant infrapopliteal disease undergoing open tibial bypass or endovascular tibial interventions to formulate a relevant comparator with the outcomes reported from BASIL-2.
Methods: The study population consisted of patients in BEST-CLI with adequate single segment saphenous vein conduit randomized to open bypass or endovascular intervention (cohort 1) who additionally had significant infrapopliteal disease and underwent tibial level intervention. The primary outcome was major adverse limb event (MALE) or all-cause death. MALE included any major limb amputation or major reintervention. Outcomes were evaluated using Cox proportional regression models.
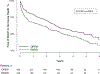
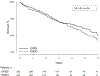
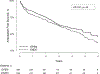
Results: The analyzed subgroup included a total of 665 patients with 326 in the open tibial bypass group and 339 in the tibial endovascular intervention group. The primary outcome of MALE or all-cause death at 3 years was significantly lower in the surgical group at 48.5% compared with 56.7% in the endovascular group (P = .0018). Mortality was similar between groups (35.5% open vs 35.8% endovascular; P = .94), whereas MALE events were lower in the surgical group (23.3% vs 35.0%; P<.0001). This difference included a lower rate of major reinterventions in the surgical group (10.9%) compared with the endovascular group (20.2%; P = .0006). Freedom from above ankle amputation or all-cause death was similar between treatment arms at 43.6% in the surgical group compared with 45.3% the endovascular group (P = .30); however, there were fewer above ankle amputations in the surgical group (13.5%) compared with the endovascular group (19.3%; P = .0205). Perioperative (30-day) death rates were similar between treatment groups (2.5% open vs 2.4% endovascular; P = .93), as was 30-day major adverse cardiovascular events (5.3% open vs 2.7% endovascular; P = .12).
Conclusions: Among patients with suitable single segment great saphenous vein who underwent infrapopliteal revascularization for chronic limb-threatening ischemia, open bypass surgery was associated with a lower incidence of MALE or death and fewer major amputation compared with endovascular intervention. Amputation-free survival was similar between the groups. Further investigations into differences in comorbidities, anatomical extent, and lesion complexity are needed to explain differences between the BEST-CLI and BASIL-2 reported outcomes.
Keywords: Amputation-free survival; BASIL-2; BEST-CLI; Chronic limb-threatening ischemia; Infrapopliteal disease; Major adverse limb events; Tibial bypass; Tibial disease; Tibial endovascular intervention.
Copyright © 2024 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: KG: advisory board for Boston Scientific.
AF: Novo Nordisk Foundation, Grant recipient; Sanifit, Consultant; LeMaitre, Consultant; BioGenCell, Consultant; Dialysis-X, Advisory Board; iThera Medical, Advisory Board
MM: Advisor Janssen
MC: Abbott Vascular DSMB
JS: Education Grant WL Gore, paid to BU; education grant from Becton Dickinson paid to BU
MV: Finnish PI in Voyager (Bayer) and Philips advisory board regarding hybrid facilities
EA: Philips: Consultant. Inari Medical: Participating in clinical trials
KR: consultant or member of a scientific advisory board for the following entities: Abbott Vascular; Althea Medical; Angiodynamics; Auxetics; Becton-Dickinson; Boston Scientific; Contego; Crossliner; Innova Vascular; Inspire MD; Janssen/Johnson and Johnson; Magneto; Mayo Clinic; MedAlliance; Medtronic; Neptune Medical; Penumbra; Philips; Surmodics; Terumo; Thrombolex; Truvic; Vasorum; Vumedi. KR owns equity or stock options in the following entities: Access Vascular; Aerami; Althea Medical; Auxetics; Contego; Crossliner; Cruzar Systems; Endospan; Imperative Care/Truvic; Innova Vascular; InspireMD; JanaCare; Magneto; MedAlliance; Neptune Medical; Orchestra; Prosomnus; Shockwave; Skydance; Summa Therapeutics; Thrombolex; Vasorum; Vumedi. KR or his institution (on my behalf) receive research grants from the following entities: NIH; Abiomed; Boston Scientific; Novo Nordisk Foundation; Penumbra; Gettinge-Atrium. Serves as a member of the Board of Directors of the following organization: The National PERT ConsortiumTM
Figures

References
-
- Lawrence PF, Gloviczki P. Global Vascular guidelines for patients with critical limb-threatening ischemia. J Vasc Surg 2019; 69: 1653–54 - PubMed
-
- Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016; 63 (suppl): 3S–21 - PubMed
-
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124: 2020–45
-
- Aboyans V, Ricco J-B, Bartelink MEL, Björck M, Brodman M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816 - PubMed
-
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial. Health Technol Assess 2010; 14: 1–210 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

