First-line treatment for femoroacetabular impingement syndrome and hip-related quality of life: study protocol for a multicentre randomised controlled trial comparing a 6-month supervised strength exercise intervention to usual care (the Better Hip Trial)
- PMID: 38908842
- PMCID: PMC11328646
- DOI: 10.1136/bmjopen-2023-078726
First-line treatment for femoroacetabular impingement syndrome and hip-related quality of life: study protocol for a multicentre randomised controlled trial comparing a 6-month supervised strength exercise intervention to usual care (the Better Hip Trial)
Abstract
Introduction: Femoroacetabular impingement syndrome (FAIS) is a motion-related and position-related clinical condition of the hip associated with pain, reduced physical function and hip-related quality of life (QoL). Interestingly, higher maximal muscle strength is associated with less pain, better physical function and improved QoL in people with FAIS. Furthermore, preliminary evidence suggests that a proportion of patients with FAIS respond positively to strength exercise as first-line treatment. Nonetheless, there is little evidence supporting a specific exercise intervention offered as a first-line treatment. We will conduct a randomised controlled trial investigating the clinical effectiveness and cost-effectiveness of a 6-month strength exercise intervention compared with usual care as first-line treatment in patients with FAIS.
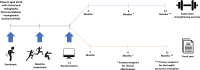
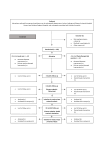
Methods and analysis: This is a multicentre randomised controlled trial that will be conducted at hospitals and physiotherapy clinics across Denmark and Australia. A total of 120 patients with FAIS will be randomised (1:1) to 6 months of supervised strength exercise or usual care. The primary outcome is the change in hip-related QoL measured using the International Hip and Outcome Tool 33 (iHOT-33) from baseline to the end of intervention. A health economic evaluation will be conducted from a societal and healthcare perspective based on the data collection over a 12-month period starting at baseline. The analysis will calculate incremental cost-effectiveness ratios using quality-adjusted life-years and iHOT-33 scores while estimating costs using microcosting and cost questionnaires. Secondary outcomes include objectively measured physical function at baseline and after 6 months and patient-reported outcomes measured at baseline, 3-month, 6-month and 12-month follow-up.
Ethics and dissemination: The trial has been approved by the Committee on Health Research Ethics in the Central Denmark Region (journal no 1-10-72-45-23) and La Trobe University Human Ethics Committee (HEC24042) and is registered at the Central Denmark Region List of Research Projects (journal no 1-16-02-115-23). Informed consent will be obtained from each participant before randomisation. Results will be published in international peer-reviewed scientific journals.
Trial registration number: NCT05927935.
Keywords: HEALTH ECONOMICS; Hip; Musculoskeletal disorders; Quality of Life; Rehabilitation medicine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK) Osteoarthritis Cartilage. 2013;21:1514–21. doi: 10.1016/j.joca.2013.07.004. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical