Integrated analysis of tertiary lymphoid structures and immune infiltration in ccRCC microenvironment revealed their clinical significances: a multicenter cohort study
- PMID: 38908856
- PMCID: PMC11331356
- DOI: 10.1136/jitc-2023-008613
Integrated analysis of tertiary lymphoid structures and immune infiltration in ccRCC microenvironment revealed their clinical significances: a multicenter cohort study
Abstract
Background: Tertiary lymphoid structures (TLSs) serve as organized lymphoid aggregates that influence immune responses within the tumor microenvironment. This study aims to investigate the characteristics and clinical significance of TLSs and tumor-infiltrating lymphocytes (TILs) in clear cell renal cell carcinoma (ccRCC).
Methods: TLSs and TILs were analyzed comprehensively in 754 ccRCC patients from 6 academic centers and 532 patients from The Cancer Genome Atlas. Integrated analysis was performed based on single-cell RNA-sequencing datasets from 21 ccRCC patients to investigate TLS heterogeneity in ccRCC. Immunohistochemistry and multiplex immunofluorescence were applied. Cox regression and Kaplan-Meier analyses were used to reveal the prognostic significance.
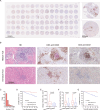
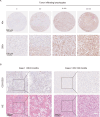
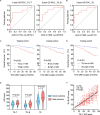
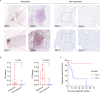
Results: The study demonstrated the existence of TLSs and TILs heterogeneities in the ccRCC microenvironment. TLSs were identified in 16% of the tumor tissues in 113 patients. High density (>0.6/mm2) and maturation of TLSs predicted good overall survival (OS) (p<0.01) in ccRCC patients. However, high infiltration (>151) of scattered TILs was an independent risk factor of poor ccRCC prognosis (HR=14.818, p<0.001). The presence of TLSs was correlated with improved progression-free survival (p=0.002) and responsiveness to therapy (p<0.001). Interestingly, the combination of age and TLSs abundance had an impact on OS (p<0.001). Higher senescence scores were detected in individuals with immature TLSs (p=0.003).
Conclusions: The study revealed the contradictory features of intratumoral TLSs and TILs in the ccRCC microenvironment and their impact on clinical prognosis, suggesting that abundant and mature intratumoral TLSs were associated with decreased risks of postoperative ccRCC relapse and death as well as favorable therapeutic response. Distinct spatial distributions of immune infiltration could reflect effective antitumor or protumor immunity in ccRCC.
Keywords: Immunotherapy; Kidney Cancer; Tumor infiltrating lymphocyte - TIL; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
