Viral modulation of type II interferon increases T cell adhesion and virus spread
- PMID: 38909022
- PMCID: PMC11193720
- DOI: 10.1038/s41467-024-49657-4
Viral modulation of type II interferon increases T cell adhesion and virus spread
Abstract
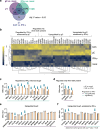
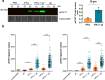
During primary varicella zoster virus (VZV) infection, infected lymphocytes drive primary viremia, causing systemic dissemination throughout the host, including the skin. This results in cytokine expression, including interferons (IFNs), which partly limit infection. VZV also spreads from skin keratinocytes to lymphocytes prior to secondary viremia. It is not clear how VZV achieves this while evading the cytokine response. Here, we show that VZV glycoprotein C (gC) binds IFN-γ and modifies its activity, increasing the expression of a subset of IFN-stimulated genes (ISGs), including intercellular adhesion molecule 1 (ICAM1), chemokines and immunomodulatory genes. The higher ICAM1 protein level at the plasma membrane of keratinocytes facilitates lymphocyte function-associated antigen 1-dependent T cell adhesion and expression of gC during infection increases VZV spread to peripheral blood mononuclear cells. This constitutes the discovery of a strategy to modulate IFN-γ activity, upregulating a subset of ISGs, promoting enhanced lymphocyte adhesion and virus spread.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Viral modulation of type II interferon increases T cell adhesion and virus spread.bioRxiv [Preprint]. 2023 May 26:2023.05.26.542397. doi: 10.1101/2023.05.26.542397. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 22;15(1):5318. doi: 10.1038/s41467-024-49657-4. PMID: 37292914 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- 443644894/Deutsche Forschungsgemeinschaft (German Research Foundation)
- AI158510/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI158510/AI/NIAID NIH HHS/United States
- R01 AI151290/AI/NIAID NIH HHS/United States
- 390874280/Deutsche Forschungsgemeinschaft (German Research Foundation)
- P30-EY08098/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI152543/AI/NIAID NIH HHS/United States
- 500627539/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 405772731/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 158989968/Deutsche Forschungsgemeinschaft (German Research Foundation)
- P30 EY008098/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

