Pivotal role of the endoplasmic reticulum stress-related XBP1s/miR-22/SIRT1 axis in acute myeloid leukemia apoptosis and response to chemotherapy
- PMID: 38909090
- PMCID: PMC11286524
- DOI: 10.1038/s41375-024-02321-8
Pivotal role of the endoplasmic reticulum stress-related XBP1s/miR-22/SIRT1 axis in acute myeloid leukemia apoptosis and response to chemotherapy
Abstract
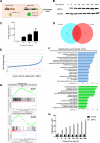
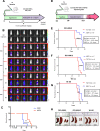
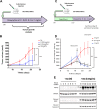
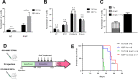
Malignant growth relies on rapid protein synthesis frequently leading to endoplasmic reticulum (ER) overload and accumulation of unfolded or misfolded protein in this cellular compartment. In the ER, protein homeostasis is finely regulated by a mechanism called the unfolded protein response (UPR), involving the activation of signalization pathways mediated by three transmembrane proteins, namely PERK, IRE1 and ATF6. IRE1 endoribonuclease activation leads in particular to the splicing of the cytosolic mRNA encoding the key UPR-specific transcription factor XBP1s. Our study shows that sustained activation of XBP1s expression in acute myeloid leukemia (AML) cells induces apoptosis in vitro and in vivo, whereas a moderate XBP1s expression sensitizes cells to chemotherapeutic treatments. ChIP-seq experiments identified specific XBP1s target genes including the MIR22HG lncRNA, the precursor transcript of microRNA-22-3p. miR-22-3p upregulation by XBP1s or forced expression of miR-22 significantly decreases cell's viability and sensitizes leukemic cells to chemotherapy. We found that miR-22-3p intracellular effects result at least partially from the targeting of the mRNA encoding the deacetylase sirtuin-1 (SIRT1), a well-established pro-survival factor. Therefore, this novel XBP1s/miR-22/SIRT1 axis identified could play a pivotal role in the proliferation and chemotherapeutic response of leukemic cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

