Reducing disparities in behavioral health treatment in pediatric primary care: a randomized controlled trial comparing Partnering to Achieve School Success (PASS) to usual ADHD care for children ages 5 to 11 - study protocol
- PMID: 38909215
- PMCID: PMC11193903
- DOI: 10.1186/s12875-024-02473-7
Reducing disparities in behavioral health treatment in pediatric primary care: a randomized controlled trial comparing Partnering to Achieve School Success (PASS) to usual ADHD care for children ages 5 to 11 - study protocol
Abstract
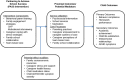
Background: Integrating behavioral health services into pediatric primary care can improve access to care, especially for children marginalized by poverty and racial/ethnic minority status. In primary care, a common presenting concern is attention-deficit/hyperactivity disorder (ADHD). Services in primary care for marginalized children with ADHD typically include medication alone; therapy to improve skills and build relationships is less available. This study evaluates the effectiveness of a behavioral intervention offered through primary care for marginalized families coping with ADHD (Partnering to Achieve School Success, PASS) compared to treatment as usual (TAU).
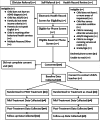
Method: Three hundred participants will be randomly assigned to PASS or TAU. Participants include children ages 5 to 11 who have ADHD and are from economically marginalized families. PASS is a personalized, enhanced behavioral intervention that includes evidence-based behavior therapy strategies and enhancements to promote family engagement, increase caregiver distress tolerance, and provide team-based care to improve academic and behavioral functioning. TAU includes services offered by primary care providers and referral for integrated behavioral health or community mental health services. Outcomes will be assessed at mid-treatment (8 weeks after baseline), post-treatment (16 weeks), and follow-up (32 weeks) using parent- and teacher-report measures of service use, child academic, behavioral, and social functioning, parenting practices, family empowerment, and team-based care. Mixed effects models will examine between-group differences at post-treatment and follow-up. Analyses will examine the mediating role of parenting practices, family empowerment, and team-based care. Subgroup analyses will examine differential effects of intervention by child clinical characteristics and socioeconomic factors.
Discussion: This study is unique in targeting a population of children with ADHD marginalized by low socioeconomic resources and examining an intervention designed to address the challenges of families coping with chronic stress related to poverty.
Trial registration: This study was registered on clinicaltrials.gov (NCT04082234) on September 5, 2019, prior to enrollment of the first participant. The current version of the protocol and IRB approval date is October 4, 2023. Results will be submitted to ClinicalTrials.gov no later than 30 days prior to the due date for the submission of the draft of the final research report to the Patient-Centered Outcomes Research Institute.
Keywords: (Three to ten) attention-deficit/hyperactivity disorder; Behavior therapy; Family engagement; Pediatric primary care; Reducing disparities.
© 2024. The Author(s).
Conflict of interest statement
Study investigators will report financial and other interests to our institution’s Office of Research Compliance on an ongoing basis. In addition, the principal investigators will report conflicts of interest to the funding agency on an ongoing basis. At the time of publication of this protocol, the authors and study team members have no competing interests to declare.
Figures
Similar articles
-
A process for developing community consensus regarding the diagnosis and management of attention-deficit/hyperactivity disorder.Pediatrics. 2005 Jan;115(1):e97-104. doi: 10.1542/peds.2004-0953. Pediatrics. 2005. PMID: 15629972
-
The effect of a family-based mindfulness intervention on children with attention deficit and hyperactivity symptoms and their parents: design and rationale for a randomized, controlled clinical trial (Study protocol).BMC Psychiatry. 2016 Mar 15;16:65. doi: 10.1186/s12888-016-0773-1. BMC Psychiatry. 2016. PMID: 26980323 Free PMC article. Clinical Trial.
-
Does the treatment of anxiety in children with Attention-Deficit/Hyperactivity Disorder (ADHD) using cognitive behavioral therapy improve child and family outcomes? Protocol for a randomized controlled trial.BMC Psychiatry. 2019 Nov 13;19(1):359. doi: 10.1186/s12888-019-2276-3. BMC Psychiatry. 2019. PMID: 31722690 Free PMC article. Clinical Trial.
-
Improving Communication about Care Goals for Children with ADHD [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Nov. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Nov. PMID: 37607239 Free Books & Documents. Review.
-
Assessing Support for Medicine Decision Making for Youth with ADHD Who Receive Therapy [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Sep. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Sep. PMID: 37672618 Free Books & Documents. Review.
References
-
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. - DOI - PMC - PubMed
-
- Shahidullah JD, Hostutler CA, Coker TR, Allman Dixson A, Okoroji C, Mautone JA. Child Health Equity and Primary Care. Am Psychol. 2023;78(2):93–106. 10.1037/amp0001064. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical