Fluorometric Analysis of Carrier-Protein-Dependent Biosynthesis through a Conformationally Sensitive Solvatochromic Pantetheinamide Probe
- PMID: 38909314
- PMCID: PMC11622929
- DOI: 10.1021/acschembio.4c00169
Fluorometric Analysis of Carrier-Protein-Dependent Biosynthesis through a Conformationally Sensitive Solvatochromic Pantetheinamide Probe
Abstract
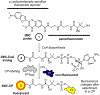
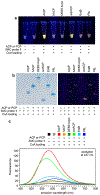
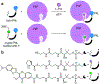
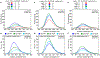
Carrier proteins (CPs) play a fundamental role in the biosynthesis of fatty acids, polyketides, and non-ribosomal peptides, encompassing many medicinally and pharmacologically relevant compounds. Current approaches to analyze novel carrier-protein-dependent synthetic pathways are hampered by a lack of activity-based assays for natural product biosynthesis. To fill this gap, we turned to 3-methoxychromones, highly solvatochromic fluorescent molecules whose emission intensity and wavelength are heavily dependent on their immediate molecular environment. We have developed a solvatochromic carrier-protein-targeting probe which is able to selectively fluoresce when bound to a target carrier protein. Additionally, the probe displays distinct responses upon CP binding in carrier-protein-dependent synthases. This discerning approach demonstrates the design of solvatochromic fluorophores with the ability to identify biosynthetically active CP-enzyme interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Evidence-based toxicology: a comprehensive framework for causation.Hum Exp Toxicol. 2005 Apr;24(4):161-201. doi: 10.1191/0960327105ht517oa. Hum Exp Toxicol. 2005. PMID: 15957536
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Lai JR; Koglin A; Walsh CT Carrier Protein Structure and Recognition in Polyketide and Nonribosomal Peptide Biosynthesis. Biochemistry 2006, 45 (50), 14869–14879. - PubMed
-
- Byers DM; Gong H Acyl Carrier Protein: Structure-Function Relationships in a Conserved Multifunctional Protein Family. Biochemistry and Cell Biology 2007, 85 (6), 649–662. - PubMed
-
- Mercer AC; Burkart MD The Ubiquitous Carrier Protein - A Window to Metabolite Biosynthesis. Nat. Prod Rep 2007, 24 (4), 750–773. - PubMed
-
- Fischbach MA; Walsh CT Assembly-Line Enzymology for Polyketide and Nonribosomal Peptide Antibiotics: Logic, Machinery, and Mechanisms. Chem. Rev 2006, 106 (8), 3468–3496. - PubMed
-
- Katz L; Baltz RH Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol Biotechnol 2016, 43 (2–3), 155–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

