Comparing the effectiveness and cost-effectiveness of text-message reminders and telephone patient navigation to improve the uptake of faecal immunochemical test screening among non-responders in London: a randomised controlled trial protocol
- PMID: 38909999
- PMCID: PMC11328611
- DOI: 10.1136/bmjopen-2023-079482
Comparing the effectiveness and cost-effectiveness of text-message reminders and telephone patient navigation to improve the uptake of faecal immunochemical test screening among non-responders in London: a randomised controlled trial protocol
Abstract
Introduction: Participation in bowel cancer screening is lower in regions where there is high ethnic diversity and/or socioeconomic deprivation. Interventions, such as text message reminders and patient navigation (PN), have the potential to increase participation in these areas. As such, there is interest in the comparative effectiveness of these interventions to increase bowel cancer screening participation, as well as their relative cost-effectiveness.
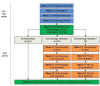
Methods and analysis: This study will use a three-arm randomised controlled trial design to compare the effectiveness and cost-effectiveness of text message reminders and PN to increase the uptake of bowel cancer screening in London. Participants will be individuals who have not returned a completed faecal immunochemical test kit within 13 weeks of receiving a routine invitation from the London bowel cancer screening hub. Participants will be randomised (in a 1:1:1 ratio) to receive either (1) usual care (ie, 'no intervention'), (2) a text message reminder at 13 weeks, followed by repeated text message reminders at 15, 17 and 19 weeks (in the event of non-response) or (3) a text message reminder at 13 weeks, followed by PN telephone calls at 15, 17 and 19 weeks in the event of non-response. The primary endpoint will be participation in bowel cancer screening, defined as 'the return of a completed kit by week 24'. Statistical analysis will use multivariate logistic regression and will incorporate pairwise comparisons of all three groups, adjusted for multiple testing.
Ethics and dissemination: Approvals to conduct the research have been obtained from University College London's Joint Research Office (Ref: 150666), the Screening Research, Innovation and Development Advisory Committee ('RIDAC', Ref: 2223 014 BCSP Kerrison), the Health Research Authority (Ref: 22/WM/0212) and the Confidentiality Advisory Group (Ref: 22/CAG/0140). Results will be conveyed to stakeholders, notably those managing the screening programme and published in peer-reviewed journals/presented at academic conferences.
Trial registration number: ISRCTN17245519.
Keywords: Mass Screening; Patient Navigation; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Cancer Research UK . Bowel Cancer Statistics | Cancer Research UK. 2019.
-
- Public Health England Public Health Profiles. 2020. https://fingertips.phe.org.uk/search/bowel%20screening#page/0/gid/1/ati/... Available.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
